Research Article
Phytochemical Analyses and Larvicidal Potentiality of Fruit Peel Extracts of Citrus limetta against Filarial Vector Culex quinquefasciatus 
2 Department of Zoology, Maharajadhiraj Uday Chand Womens' College, Burdwan, West Bengal, India
3 Department of Zoology, University of Gour Banga, Malda, West Bengal, 732103, India
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2016, Vol. 6, No. 22 doi: 10.5376/jmr.2016.06.0022
Received: 18 Jul., 2016 Accepted: 24 Nov., 2016 Published: 29 Dec., 2016
Mallick S., Adhikari U., Rawani A., and Chandra G., 2016, Phytochemical analyses and larvicidal potentiality of fruit peel extracts of Citrus limetta against filarial vector Culex quinquefasciatus, 6(22): 1-7 (doi: 10.5376/jmr.2016.06.0022)
Different concentrations of crude and different solvents viz., n-hexane, ethyl acetate, and methanol fruit peel extracts of Citrus limetta Risso (C. limetta) were taken for investigation for larvicidal activity against Culex quinquefasciatus Say, 1823 (Cx. quinquefasciatus) mosquito species. Crude extract showed good larvicidal activity with very low concentrations against 1st - 4th instars larvae. Only 3rd instar larvae were taken for larvicidal bioassay experiments for each solvent extract. LC50 values of n-hexane, ethyl acetate, and methanol fruit peel extracts of C. limetta were 661.27, 1268, and 939.43 ppm respectively after 24 h of exposure. No mortality was observed on control treatments. Log probit analyses, regression equations, and R2 values of larvicidal bioassay experiments with crude and different solvent extracts were determined. Statistical justification was done through ANOVA analyses. Qualitative phytochemical analyses were carried out and detected different secondary metabolites. No mortality was observed on tested non target organisms.
Introduction
Many dreadfull diseases of human beings like filariasis, malaria, dengue/dengue haemorrhagic fever, Japanese encephalitis etc are transmitted by different mosquito species and cause million of death every year. Mosquitoes not only transmit parasites and pathogens but also cause allergic reactions that include local skin and systemic sensitivity (Mallick et al., 2015a; Pedro et al., 2014). Cx. quinquefasciatus Say, 1823 transmits lymphatic filariasis of human and also plays major role in transmitting avian malaria (Bockarie et al., 2009). Lymphatic filariasis is a neglected tropical disease. In 2000, 40 million people disfigured and incapacited out of over 120 million filariasis infected people worldwide. Currently preventive chemotherapy is needed to stop the spread of infection in the area where 947 million people in 54 countries are living (WHO, 2016). Synthetic mosquito larvicides were used to control mosquito population in most parts of the world but those are toxic to human body, animal life, plants and emergence of resistance in mosquitoes, especially to pyrethroids, organochlorine and organophosphate compounds. To kill larvae in their habitats is the primary and foremost step to control mosquito population (Hayatie et al., 2015; Singh et al., 2015). Variety of small organic molecules are produced by plants called secondary metabolites that protect plants from pathogens, herbivores or competitors and these secondary metabolites can be divided into different chemical groups such as alkaloids, terpenoids, phenolics, plant amines, glycosides etc (Bilal et al., 2012). Therefore, plants extracts are alternative source of many bioactive chemicals that are nontoxic and biodegradable and therefore their uses are most suitable to control mosquito population (Velu et al., 2015; Singha Ray et al., 2014; Bhattarcharya and Chandra, 2014). Many researchers worked with many plants for their larvicidal activities against different mosquito species (Mallick et al., 2014; Mallick et al., 2015b; Mallick et al., 2016; Mallick and Chandra, 2015a; 2015b; 2015c; Mallick and Chandra, 2016; Singha and Chandra, 2011; Bhattarcharya and Chandra, 2013; Singha et al., 2011). C. limetta belongs to genus Citrus and family Rutaceae. Common name of many varieties of C. limetta includes Mediterranean sweet lemon, sweet lime, and sweet limetta. In India it is called sathukudi in Tamil, mosambi, musambi or mousambi in Hindi and Urdu. It is named as malta in Bangladesh. It is a small tree (average 8 m in height) with irregular branches, smooth brownish-grey bark and numerous thorns (Mohammed et al., 2013). In traditional indigenous medicinal system, its juice is used for curing jaundice, malaria, and fever. Essential oils are obtained from citrus peels and these oils are considered as the potential sources for screening of antimicrobial, antioxidant, anticancer and free radical scavenging agents (Javed et al., 2013). An ecofriendly approach to control larvae of Cx. quinquefasciatus mosquito with discarded materials like fruit peel extracts of C. limetta was carried out during present experiment.
1 Results
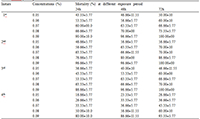
Percent mortality of 1st - 4th instars larvae of Cx. quinquefasciatus by aqueous fruit peel extract of C. limetta (at different concentrations) are presented in Table 1. Percent mortality increased with increase in concentration and time of exposure. No mortality was observed on negative control treatments. Table 2 depicts the percent mortality at different concentrations of each of different solvent crude fruit peel extract of C. limetta at different time periods and percent mortality increased with increase in concentrations of each solvent crude fruit peel extract and exposure period. No mortality was noticed on ethanol treated control. Table 3 and Table 4 demonstrated LC50, LC90, regression equations, R2 values of aqueous and different solvent crude extracts respectively. LC50 and LC90 values gradually decreased on exposure periods. The percent mortality (Y) was positively correlated with the concentration (X) of the extracts as coefficient of determination values close to 1 in each case (Table 3 and Table 4). LC50 and LC90 values were lower in the order of hexane< methanol< ethyl acetate crude fruit peel extract. Qualitative phytochemical analyses revealed the presence of alkaloids, flavonoids, terpenoids and steroids but absence of tannins and phenols (Table 5). No mortality or sluggishness was noted on tested non target organisms like, Chironomus circumdatus larvae and tadpoles of Bufo melanostictus. Three ways ANOVA analyses were done, using all instars larvae, concentrations of aqueous peel extract and times of exposure as independent variables and percent mortality of larvae of Cx. quinquefasciatus as dependent variable. Percent mortality showed statistically significance (p< 0.05) in terms of instars, concentrations and time of exposures. But there was no significant value of percent mortality of the larvae when the interactions of the variables are considered except interaction of the Instars of Cx. quinquefasciatus and concentrations of the extract aqueous peel extract (Table 6).
|
Table 1 Percent mortality of different instars of Culex quinquefasciatus exposed to different concentrations of aqueous peel extract of Citrus limetta (Mean percent mortality ± Standard deviation) |
|
Table 2 Percent mortality of 3rd instar larvae of Culex quinquefasciatus exposed to different concentrations of solvent crude peel extracts of Citrus limetta (Mean percent mortality ± Standard deviation) |
|
Table 3 Log probit analyses and regression analyses of larvicidal activity of aqueous peel extract of Citrus limetta against different larval instar forms of Culex quinquefasciatus. (Y= percent mortality, X= concentration, LC= Lethal Concentration) |
|
Table 4 Log probit analyses and regression analyses of larvicidal activity of different solvent crude peel extracts of Citrus limetta against 3rd larval instar of Culex quinquefasciatus (Y= mortality percent, X= Concentration, LC= Lethal Concentration) |
|
Table 5 Result of qualitative analyses of phytochemicals from fruit peel extract of Citrus limetta |
|
Table 6 Completely randomized three way ANOVA analyses using all instars (I) of Culex quinquefasciatus, hour (H), and Concentrations (C) of aqueous crude peel extract of Citrus limetta as three independent parameters |
2 Discussions
Kumar et al. (2012) reported the mosquito larvicidal efficacy of n-hexane and petroleum ether solvent peel extract of C. limetta against 4th instar larvae of Indian strains of Anopheles stephensi (An. Stephensi) and Aedes aegypti (Ae. aegypti). In the present piece of work, the efficacy of aqueous and n-hexane, ethyl acetate and methanol solvent crude fruit peel extracts of C. limetta has been evaluated against Cx. quinquefasciatus. Many authors reported the role of fruit peel extracts of different plants against different mosquito species. Velu et al. (2015) worked with Arachis hypogaea for its larvicidal activity against 4th instar larvae of Ae. aegypti and An. stephensi upto 24 h of exposure. Cent percent mortality was noted at 150 ppm concentration and LC50 values were 45.75 and 45.98 ppm against Ae. aegypti and An. stephensi respectively. Murugan et al. (2012) reported the larvicidal activity of ethanolic fruit peel extract of Citrus sinensis against different mosquito species upto 24 h of exposure period, of which LC50 values of 1st to 4th instars larvae of Cx. quinquefasciatus were 244.70, 324.04, 385.32 and 452 .78 ppm respectively. The larvicidal activity of fruit peel extract of C. sinenses against An. subpictus having LC50 and LC90 values were 53.80 and 32.52 ppm after 24 and 48 h of exposures which were reported by Bhagavan et al. (2009). Among tested three solvent fruit peel extracts of C. limetta, n-hexane showed best larvicidal activity against 3rd instar Cx. quinquefasciatus larvae. Non-target organisms did not show any mortality and sluggishness with tested concentrations of n-hexane solvent fruit peel extract of C. limetta. Different secondary metabolites are present in fruit peel extracts of C. limetta. These secondary metabolites are probably responsible for larvicidal activity of Cx. quinquefasciatus mosquito species. So tested aqueous as well as n-hexane solvent crude fruit peel extracts of C. limetta serve as good larvicidal agents against Cx. quinquefasciatus mosquito species. Active chemical compound (s) involved in n-hexane fruit peel extract of C. limetta are to be further investigated. Fruit peel is a waste product so its use to control mosquito will also be cost effective.
3 Materials and methods
3.1 Collection of fruits
Fruits of C. limetta were collected during the month of September, 2014 from the market of Burdwan town, West Bengal, India (23◦16ꞌ N, 87◦54ꞌ E). Collected fruits were washed thoroughly with running tap water and thereafter rinsed with distilled water. They were dried on paper towel with gentle blowing of air. Peels were separated from fruits by steel knife. Spongy tissues of peels were discarded.
3.2 Test mosquito
Larvae of Cx. quinquefasciatus were collected from drains of Burdwan town and kept in a plastic tray with tap water. Larvae were identified properly after rearing into adult stage following the Key of Christopher (1933) and Barraud (1934). Larvae of Cx. quinquefasciatus were well maintained in the laboratory under 27± 2◦ C temperature and 85% relative humidity. The larvae were provided mixtutre of dog biscuits and dried Brewer’s yeast powdered in the ratio 3:1. Pupae were transferred from plastic tray to glass beaker (500 ml) containing tap water and the beaker containing pupae was kept in mosquito cage (30×30×30 cm3) where adults were emerged. Adults were provided initially with glucose solution (10%) in a plastic bowl with a cotton wick. Adults were provided blood meal from restrained pigeon on day five. Two Plastic bowls with 100 ml of tap water were kept in the cage for oviposition. Next generation larvae were used for larvicidal bioassay experiments.
3.3 Preparation of crude extract
Washed and cleaned dried fruit peels of C. limetta were crushed by electrical grinder and juice was filtered through muslin cloth and filtrate was taken on a beaker and the filtrate was used as a stock aqueous test solution. From stock crude test solution, different concentrations (i.e. 0.05, 0.06, 0.07, 0.08, and 0.09%) of aqueous test solutions were prepared through dilution with tap water for larvicidal bioassay experiments.
3.4 Preparation of solvent crude extracts
Fruit peels of C. limetta were cut into very small pieces by steel knife and dried in shade for 13-15 days. 200 g dried peels of C. limetta were placed into the thimble of Soxhlet apparatus and 2000 ml of each solvent viz., n-hexane, ethyl acetate, and methanol were passed, using fresh sample for each extraction with the solvent. The extraction period was 72 h with each solvent. Extractives of each solvent was collected separately, filtered through Whatman No. 1 filter paper and concentrated by evaporation. Three semisolid extractives were stored separately at 4º C in a refrigerator for further experiments. 5 gm of each of three solvent crude extracts was initially dissolved in 5 ml of ethanol and then added 45 ml of distilled water to prepare 50 ml of stock test solution. Taking calculated volume of each stock test solutions on plastic bowl (225 ml capacity and 9 cm in diameter) by pipette and adding required volume of tap water on it, required graded concentrations i.e. 200, 400, 600, 800, and 1000 ppm was made for each solvent crude extract.
3.5 Larvicidal bioassay
The larvicidal bioassays were done following the standard protocol of World Health Organization, (2005) with slight modification in Parasitology Laboratory. All instars larvae were used in bioassay experiments with aqueous extract and only 3rd instar larvae were used in bioassay experiments with each solvent crude extract. Thirty larvae were put in each plastic bowl containing 100 ml of test solution of different concentrations of aqueous (0.05-0.09%) and different concentrations (i.e. 200, 400, 600, 800, 1000 ppm) of each of n-hexane, ethyl acetate and methanol fruit peel extract of C. limetta. Fresh stock solution of aqueous and each of different solvent crude extracts were prepared on the same day of larvicidal bioassay experiments. Bio assay experiments (for each replica) with aqueous extract for all instars larvae were done concurrently. 100 ml of tap water with 0.5 ml of ethanol was used as ethanol treated control and only 100 ml of tap water was used as negative control. Larval percent mortality was recorded after 24, 48, and 72 h of post exposures cumulatively. Dead larvae were identified when they could not move after touching with fine brush in the siphon or cervical region. All experiments were replicated three times on separate three days under laboratory conditions at 25º- 30º C and 80- 90% relative humidity.
3.6 Phytochemical analyses of fruit peel extracts of C. limetta
Qualitative phytochemical analyses of shade dried fruit peel extracts (ethanol and aqueous) were done according to Trease and Evans (1989) with slight modification.
3.6.1 Test of terpenoids (Salkowski test)
4 ml of ethanol extract was taken in a clean test tube and 2 ml of chloroform was added firstly and thereafter added 3 ml of concentrated H2SO4 slowly through the interior wall of test tube. A reddish brown coloration of the interface was developed which indicate the presence of terpenoids.
3.6.2 Test of alkaloids
4 ml of ethanol extract was taken in a test tube and added 2 drops of 2N HCL and thereafter added 2 drops of Mayer’s reagent. Pale yellow color precipitation is the indication of presence of alkaloids in the sample.
3.6.3 Test of steroids
4 ml of ethanol extract was taken in a clean test tube and 2 ml of concentrated H2SO4 was added carefully. A brown color ring will develop which indicate the presence of steroids.
3.6.4 Test of flavonoids
4 ml of aqueous extract was taken in a clean test tube and was added 2-3 drops of NaOH solution. Intense colors will develop which become colorless if added dilute HCL. This is the indication of presence of flavonoids in the sample.
3.6.5 Test of tannins and phenols
4 ml of aqueous extract was taken in a clean test tube and was added 1 ml of ferric chloride solution. The color of the solution change into blue green indicates the presence of tannin and phenolic compounds in the sample.
3.7 Effect of n-hexane solvent crude fruit peel extracts of C. limetta on non-target organisms
LC50 value of n-hexane solvent crude peel extracts of C. limetta against 3rd instar larvae of Cx. quinquefasciatus after 24 h of exposure were used for observation to the effect on non-target organism. Chironomus circumdatus larvae and tadpoles of Bufo melanostictus were tested as non-target organisms as described by mallick et al. (2015a). Twenty 4th instar Chironomous circumdatus larvae, twenty tadpoles of Bufo melanostictus were kept separately in 500 ml of glass beaker containing 200 ml of test solution with dose metionted. For the preparation of test solutions, pond water was used. Data of mortality of non target organisms were noted after 24, 48, and 72 h of post exposures. Control experiments for each organism were run parallel on a 500 ml beaker containing 200 ml of pond water with 0.5 ml of ethanol. All experiments were done three times on separate three days.
3.8 Statistical analyses
Percent mortalities and standard deviations were calculated through ‘MS EXCEL 2007’. ‘Stat Plus v5 (trial version)’software were used to calculate LC50, LC90 values through Log-probit analyses (95% confidence level), regression equations (X- concentrations, Y- mortality percent), R2 (Co efficient of determination) values and ANOVA for testing statistically significance.
Authors’ contributions
SM performed the larvicidal bioassay experiments with aqueous and different crude solvent extracts of Citrus limetta and prepared the manuscript. UA has done the phytochemical analyses of fruit peel extracts of Citrus limetta. A Rawani has done the experiment of effect of n-hexane solvent fruit peel extract of Citrus limetta on non-target organisms. GC supervised the whole work and corrected the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Authors are much thankful to Professor Dr. A. Mukhopadhyay, Department of Botany, The University of Burdwan, Burdwan, West Bengal, India for identification of the plant species. We are also grateful to UGC DRS for providing financial support.
Bagavan A., Kamaraj C., Rahuman A., Elango G., Zahir A.A., and Pandiyan G., 2009 Evaluation of larvicidal and nymphicidal potential of plant extracts against Anopheles subpictus Grassi, Culex tritaeniorhynchus Giles and Aphis gossypii Glover, Parasitology Research., 104: 1109-17
Barraud P.J., 1934, The Fauna of British India, including Ceylon and Burma, Diptera Vol -IV. Taylor and Francis London: pp.1-455
Bilal H., Akram W., and Hassan S.A., 2012, Larvicidal activity of Citrus limonoids against Aedes albopictus larvae, Journal of Arthropod Borne Diseases., 6(2): 104-111
Bockarie J.M., Erling M.P., Graham B.W., and Michael E., 2009, Role of vector control in the global program to eliminate lymphatic filariasis, Annual Review of Entomology., 54: 469-487
Bhattacharya K. and Chandra G., 2014, Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrata L. (Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae), Asian Pacific Journal of Tropical Disease., 4 (Suppl 1): S226-S232
http://dx.doi.org/10.1016/S2222-1808(14)60444-8
Bhattacharya K., and Chandra G., 2013, Bioactivity of Acyranthes aspera (Amaranthaceae) Foliage against the Japanese Encephalitis Vector Culex vishnui Group, Journal of Mosquito Research., 3(13): 89-96
Christophers S.R., 1933, The Fauna of British India, including Ceylon and Burma, Diptera Vol -V. Taylor and Francis London: pp.360
Hayatie L., Biworo A., and Suhartono E., 2015, Aqueous extracts of seed and peel of Carica Papaya against Aedes aegypti, Journal of Medical and Biological Engineering., 4(5): 417-421
Javed S., Ahmad R., Shahzad K., Nawaz S., Saeed S., and Saleem Y., 2013, Chemical constituents, antimicrobial and antioxidant activity of essential oil of Citrus limetta var. Mitha (sweet lime) peel in Pakistan, African Journal of Microbiology Research., 7(24): 3071-3077
Kumar S., Warikoo R., Mishra M., Seth A., and Wahab N., 2012, Larvicidal efficacy of the Citrus limetta peel extracts against Indian strains of Anopheles stephensi Liston and Aedes aegypti L, Parasitology Research., 111(1): 173-8
Mallick S. and Chandra G., 2016, Larvicidal efficacy of root and stem bark extracts of the plant, Annona reticulata against filarial vector, Culex quinquefasciatus Journal of Mosquito Research, 6(3): 1-8
Mallick S., and Chandra G., 2015a, larvicidal, pupicidal, oviposition deterrent activity and smoke toxicity of mature leaf extracts of Annona reticulata Linn. against filarial vector Culex quinquefasciatus Say, International Journal of Pharma and Bio Sciences, 6(4): (B): 244-253
Mallick S., and Chandra G., 2015b, Larvicidal potentiality of root extracts of Annona reticulata Linn. against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae), Journal of Mosquito Research., 5(10): 1- 7
Mallick S., and Chandra G., 2015c, Larvicidal activities of extracts of stem bark of Annona reticulata against filarial vector Culex quinquefasciatus, International Journal of Pharma and Bio Sciences., 6(3): (B): 1347-1356
Mallick S., Banerjee R., and Chandra G., 2015a, Mosquito larvicidal potential of ethanol leaf extract of the plant, Annona reticulata L. against Aedes aegypti L. and Culex quinquefasciatus Say (Diptera: Culicidae), Journal of Mosquito Research, 5(19): 1-7
Mallick S., Bhattacharya K., and Chandra G., 2014, Mosquito larvicidal potentiality of wild turmeric, Curcuma aromatica rhizome extracts against Japanese Encephalitis vector Culex vishnui group, Journal of Mosquito Research, 4(19): 1-6
Mallick S., Mukherjee D., and Chandra G., 2015b, Evaluation of larvicidal efficacy of acetone leaf extracts of Annona reticulata Linn. against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae), Journal of Mosquito Research, 5(9): 1-7
Mallick S., Mukherjee D., Singha Ray A., and Chandra G., 2016, Larvicidal efficacy of fruit peel extracts of Citrus maxima against Culex quinquefasciatus, Journal of Mosquito Research, 6(20): 1-8
Mohammed A.S., Mohammed M.U.R., and Mohammed R.A.T., 2013, Investigation of cytotoxic potential of ethanolic extract of Citrus limetta fruit peel, Paederia foetida leaves and methanolic extract of Cuscuta reflexa, Journal of Medical Plants Studies., 1(1): 34-37
Murugan K., Kumar P.M., Kovendan K., Amersan D., Subrmanium J., and Hwang J., Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitology Research,111(4):1757-69, doi 10.1007/s00436-012-3021-8
Pedro M.G.J., Aubrey N.A., Bryle A.L.E., and Maria F.L.S., 2014, Larvicidal activity of selected plant extracts against the dengue vector Aedes aegypti mosquito, International Research Journal of Biological Sciences. 3(4): 23-32
Singh A., Bhattacharya K., and Chandra G., 2015, Efficacy of Nicotiana Plumbaginifolia (Solanaceae) leaf extracts as larvicide against malarial vector Anopheles Stephensi Liston 1901, International Journal of Pharma and Bio Sciences., 6(1): (B) 860- 868
Singh Ray A., Bhattacharya K., Singh A., and Chandra G., 2014, Larvicidal Activity of Nelumbo nucifera Gaertn. (Nymphaeaceae) against Anopheles stephensi (Liston 1901) and its Effect on Non-target Organisms, Journal of Mosquito Research., 4(10): 1-7
Singha S., Adhikary U., and Chandra G., 2011, Smoke repellency and mosquito larvicidal potentiality of Mesua ferra L. leaf extract against filarial vector Culex quinquefasciatus Say, Asian Pacific Journal Tropical Biomedicine., 1(Suppl.1): 119-123
Singha S., and Chandra G., 2011, Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi, Asian Pacific Journal Tropical Medicine., 4(4): 288-93
Stat Plus v5, AnalystSoft Inc. - Statistical analysis program (www.analystsoft.com)
Trease G.E. and Evans W.C., 1989, Pharmacognosy, 11th Edition, Brailliar Tridel and Macmillan Publishers, London
Velu K., Elumalai D., Hemalatha P., Babu M., Janaki A., and Kaleena P.K., 2015, Phytochemical screening and larvicidal activity of peel extracts of Arachis hypogaea against chikungunya and malarial vectors, International Journal of Mosquito Research., 2 (1): 01-08
World Health Organization, 2005, Guidelines for laboratory and field testing of mosquito larvicides. Geneva: WHO (WHO/CDS/WHOPES/GCDPP/2005.13)
World Health Organization, Lymphatic filariasis, Fact Sheet, updated February 2016
. PDF(0KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Subrata Mallick
. Utpal Adhikari
. Anjali Rawani
. Goutam Chandra
Related articles
. Citrus limetta
. Culex quinquefasciatus
. Larvicide
. Non-target organisms
. Phytochemicals
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)
.png)


