Esterase profile changes in ladybeetle, Hippodamia variegata Goeze (Coleoptera: Coccinellidae) and its prey, Aphis fabae Scop. (Homoptera: Aphididae) affected by two insecticides, thiamethoxam and pirimicarb 
 Author
Author  Correspondence author
Correspondence author
Molecular Entomology, 2015, Vol. 6, No. 2 doi: 10.5376/me.2015.06.0002
Received: 12 Jan., 2015 Accepted: 28 Feb., 2015 Published: 29 Mar., 2015
Rahmani and Bandani, 2015, Esterase profile changes in ladybeetle, Hippodamia variegata Goeze (Coleoptera: Coccinellidae) and its prey, Aphis fabae Scop. (Homoptera: Aphididae) affected by two insecticides, thiamethoxam and pirimicarb, Molecular Entomology, Vol.6, No.2 1-10 (doi: 10.5376/me.2015.06.0002)
Black bean aphid, Aphis fabae Scopoli (Homoptera: Aphididae), is a cosmopolitan and serious pest of diverse plant species. In addition to inflicting direct damage, it is an important vector of viruses producing indirect damages. Hippodamia variegata, is considered as aphids predator both in their larval and adult stages. Thus, the aim of the current study was to investigate toxicological, biochemical and physiological effects of two widespread used pesticides against aphids including pirimicarb and thiamethoxam on third instar larvae of the ladybeetle and the adult female of the aphid in laboratory condition. Bioassay showed that LC50 value in thiamethoxam treatment against A. fabae and H. variegata was 113.85 and 788.55 mg (ai)L-1, respectively and LC50 value for pirimicarb treatment against two insects was 2.94 and 2740.07 mg (ai)L-1, respectively. Enzyme assays showed that AChE and CbE (evaluated by a-NA) of A. fabae inhibited significantly by pirimicarbtreatments(P>0.05), whilst thiamethoxam treatment did not affect both enzymes. Both insecticides did not affect H. variegata AChE and CbE activities, significantly. Polyacrylamide gel electrophoresisof general esterases of A. fabae by a solution mixture of both substrates (a- and b-NA) showed that only one band present in the control and in the treated insects. However, in H. variegata larvae, there were four esterase isoforms in the control and the isoforms showed changes after insect treatment with the insecticides. Thus it is concluded that susceptibility of the two insects toward the two insecticides are different and these differences were obvious in their enzyme activity and their isoforms as well.
Introduction
Aphids are a diverse group of herbivore insect pests causing direct and indirect damage to plant species including sap sucking, transmission of viruses, and secretion of honeydew (Kennedy et al. 1962; Raboudi et al. 2002; Diehl et al., 2013).
Black bean aphid, Aphis fabae Scolpoli (Homoptera: Aphididae), is a serious pest in all nymphal stages and adult (Van Emden and Harrington, 2007) that attacks almost 200 host plant species including vegetables, fruit trees and ornamentals causing damage and reducing the market value by sucking the sap from the tender shoots, pods, flowers (Volk and Stechmann 1998; Arocca et al., 2011). It is an important vector of viruses especially on sugar beets, transmitting more than 30 viruses, mainly in the nonpersistent group (Van Emden and Harrington, 2007).
Coccinellid beetles are considered as biological control agents of many injurious insects like aphids with a great economic importance in agro-ecosystem (Agarwala and Dixon 1992; Diehl et al., 2013). They are of a great value as aphids predators both in their larval and adult stages (Hippa et al. 1978; Kring et al. 1985; Gardiner et al., 2012). Hippodamia variegata (Goeze) originated in the Palearctic region (Gordon 1987). It is a widespread predator of aphids in many parts of the world (Franzmann 2002; Kontodimas and Stathas, 2005) and many agricultural ecosystems such as wheat, tobacco, cotton, vegetable and orchards (Yang et al. 1997; Wu et al., 2010; Honek et al., 2014).
This species is considered as the most important natural enemies of aphids, coccids and other soft bodied insects such as psyllids, white flies, lepidopteran insects, mealy bug (Franzman 2002). In IPM programs the integration of chemical and biological controls is a common practice and indeed the exact concept of IPM is developed as a result of applying compatible pesticides with biological control (Elzen, 2001). Application of pesticides in IPM programs could lead to both lethal and sublethal effects on arthropods, thus in addition to death, they can adversely affect life parameters (Rahmani and Bandani, 2013).
Among pesticides, pirimicarb (a carbamate chemical) is a selective insecticide used mostly for aphid control (Dewar et al., 2014; Hughes et al., 2014) which acts as acetylcholinterase inhibitor. Moreover, in recent years, neonicotinoid-class insecticides, as agonists on nicotinic acetylcholine receptors that mimic the mode of action of nicotine in postsynaptic nerve membranes, are used for the control of aphids (Chao et al., 1997; Nauen et al., 1998). Thiamethoxam, a neonicotinoed insecticide, presently is an effective chemical for the control of sucking pests such as aphids, whiteflies, thrips, some microlepidoptera, and a number of coleopteran species (Sharma and Lal, 2002).
Exposure to pesticides may result in both behavioral and physiological changes in individuals especially when individual exposed to sublethal concentrations. The physiological changes caused by sublethal concentrations of insecticides can reduce developmental rate, longevity, fecundity, and fertility (Stark and Banks, 2003; Rahmani and Bandani, 2013). However, in IPM (Integrated Pest Management) program application of selective pesticide in order to maintain beneficial species populations is mandatory. There are reports of the differential insecticide susceptibility of prey and predator (Wu and Miyata, 2005; Booth et al., 2007; Karunaratne et al. 2007; Lima et al., 2013), which is as a result of physiological and biochemical differences between the prey and its predator to detoxify insecticides. Yu (1987, 1988) examined selectivity relationships between predator and prey by comparing detoxification enzyme systems and reported that the predator showed more detoxifying enzyme activity than its prey. Biochemical changes after sublethal exposure to pesticides can be measured using specific biomarkers (like general esterases and acetylcholinesterase) that provide a measure of effects, e.g., “fitness” of the survivors (McCarthy and Shugart 1990; Kumral et al., 2011).
Among different enzymes involved in detoxification process, acetycholinesterases (AChE, EC 3.1.1.7.) and general esterases (CarEs, EC 3.1.1.1) play essential role in these process (Baffi et al 2005). General esterases are a large and diverse group of hydrolases that hydrolyze numerous substrates including esters and certain non-ester compounds (Walker and Mackness, 1983). They are commonly classified into three types based on their interactions with OP compounds (Aldridge, 1993). The A-esterases are not inhibited by OPs but degrade these insecticides as their substrates, whereas the B-esterases are readily inhibited by OPs (Aldridge, 1953). The third type the C-esterases, that was later added to the classification, do not interact with OPs (Bergmann et al., 1957). The insect esterases can either cause broad spectrum resistance to various insecticides through rapid binding and slow turnover of insecticide molecules (sequestration) or cause narrow spectrum resistance to very restricted range of insecticides containing a common ester linkage such as malathion through rapid metabolism of the insecticides (Karunaratne et al, 1995; Herath et al., 1987; Zhu et al., 2011). Enhanced production of carboxylesterases in aphids is one of the most important mechanism of resistance to the organophosphate, carbamate, and to some extent to pyrethroid insecticides (Bass et al., 2014).
Acetylcholinesterase represents a biomarker of neurotoxicity widely used for identifying exposure to anticholinesterase chemicals such as organophosphorous (OP) and carbamate (C) insecticides. Acetylcholinesterase is an important enzyme responsible for the rapid hydrolyses of acetylcholine at the cholinergic synapses, thus allowing precise control and modulation of the neural transmission (Badiou et al 2008; Bass et al., 2014).
The aim of the current study was to evaluate susceptibility of both prey (Aphis fabae) and predator (Hippodamia variegata) to two insecticides, thiamethoxam and pirimicarb, using lethal and sublethal assays. Then effects of sublethal concentrations of the insecticides on AChE and CbEs of the two species are evaluated qualitatively (in gel assays) and quantitatively (enzyme assays).
1 Results
1.1 Lethal toxicity bioassays
Bioassays showed that thiamethoxam using topical application on ladybeetle had LC10, LC30 and LC50 values of 48.21, 251.31 and 788.55 mg (ai)L-1, respectively (Table 1). This pesticide when assayed on A. fabae (using residual assays) had LC10, LC30 and LC50 values of 13.85, 48.07 and 113.85 mg (ai)L-1, respectively (Table 1).
|
Table 1 Toxicity of thiamethoxam to the third instars of Hippodamia variegata and wingless female adults of Aphis fabae after 24 hours. Note: n: total number of insects tested; LC: lethal concentration (mg (ai) L−1 ); CL: confidence limits |
Primicarb insecticide in topical apliaction against the ladybeetle had LC10, LC30 and LC50 values of 652.13, 1522.86 and 2740.07 mg (ai)L-1, respectively (Table 2) but in residual assays against the aphid had LC10, LC30 and LC50 values of 0.93, 1.84 and 2.94 mg (ai)L-1, respectively (Table 2).
|
Table 2 Toxicity of pirimicarb to the third instars of Hippodamia variegata and wingless female adults of Aphis fabae after 24 hours. Note: n: total number of insects tested; LC: lethal concentration (mg (ai) L−1 ); CL: confidence limits |
1.2 Microplate assay of AChE activity
In vivo assays showed that in A. fabae, AChE was inhibited significantly by increasing concentration of pirimicarb (df=8, 3, F=9.31, P=0.0055) (Figure 1a). As shown in Figure the effect of the LC50 dose was greater than the other two doses (LC10 and LC30). Thiamethoxam did not affect significantly AChE of the aphid (df=8, 3, F=3.57, P=0.066) (Figure 1b). However, as seen in the figure a slight effect was seen although the effect was not significant.
|
Figure 1 Effect of different concentrations of pirimicarb and thiamethoxam on AChE of Aphis fabae (a, b) and Hippodamia variegata (c) |
H. variegata AChE was not inhibited significantly by pirimicarb or thiamethoxam treatments (df=12, F=0.82, P=0.5596) (Figure 1c).
1.3 Microplate assay of General esterase activity
General esterase activity of the aphid was inhibited pirimicarb treatments significantly when alpha-NA was used as a substrate (df=8, 3, F=8.89, P=0.0061) (Figure 2a). However, pirimicarb did not affect esterase activity when beta-NA was used as a substrate (df=8, 3, F=2.40, P=0.1433) (Figure 3a). Thiamethoxam did not affect general esterase of the aphid when either substrate was used (Figure 2b and Figure 3b) (df=8, 3, F=3.28, P=0.079, for alpha-NA; df=8, 3, F=1.64, P=0.2558, for beta-NA).
|
Figure 2 Effect of different concentrations of pirimicarb and thiamethoxam on CbEs of Aphis fabae (a, b) by a-NA substrate |
General esterase activity of the ledaybeetle (H. variegate) was not affected by both insecticides, pirimicarb and thiamethoxam (beta-NA substrate, df=12, F=1.92, P=0.1643) (Figure 3c). Esterase did not have any measurable data when alpha-NA was used in ladybeetle.
|
Figure 3 Effect of different concentrations of pirimicarb and thiamethoxam on CbE of Aphis fabae (a, b) and Hippodamia variegata (c) by b-NA substrate |
1.4 Polyacrylamide gel electrophoresis (PAGE)
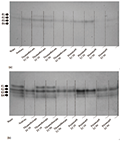
24-hour post-treated insect with two insecticides were subjected to electrophoresis in order to assess their general esterases using mixture of both substrates. Results showed that in the aphid only one band observed in the gel both in the treated insect by either insecticides (pirimicarb or thiamethoxam) and in the control insects (Figure 4a, Figure 4b).
|
Figure 4 Effect of different concentrations of pirimicarb and thiamethoxam on CbEs of Aphis fabae (a, b) showing by mixture of a- and b-NA substrates |
PAGE of the 24-hour post-treated third instar larvae of H. variegata using alpha and beta-NA substrates separately showed that there were four esterase isoforms (Figure 5a and Figure 5b). Theses isozymes were clearer in beta-NA than the other substrate (alpha-NA). Electrophoresis indicated both insecticides have some effects on the general esterases of the coccinellid larvae (Figure 5a and Figure 5b).
|
Figure 5 Effect of different concentrations of pirimicarb and thiamethoxam on CbEs of Hippodamia variegata, showing by of a-NA (a) and b-NA (b) substrates |
2 Discussion
In order to have a suitable integrated pest management programs, it is necessary to have information regarding lethal and sublethal effects of pesticides used in the agro-ecosystem on both the target insect and its predators. When pesticides are applied against target insect, beneficial arthropods such as ladybeetles also are exposing directly to the pesticides (Karunaratne et al. 2007; Jalali et al., 2009), however indirect contact with the chemical residues on plant surfaces or ingestion of pesticide-contaminated prey or hosts are the other threats to this organisms (Jepson, 1989).
Thiamethoxam and especially pirimicarb are applied in agro-ecosystems in order to control pests where a diverse range of biological agents are working to control pests. The results obtained in the current study on the effect of these two pesticides (thiamethoxam and pirimicarb) on ladybeetle, Hippodamia variegata and it's pray, Aphis fabae showed that In our study, LC50 obtained from both chemicals on H. varigata were almost 10 times higher than recommended concentration for aphids which is in congruent with the other findings (Youn et al., 2003). It was shown that LC50 values of thiamethoxam for the third larval instar of Harmonia axyridis (Colep.: Coccinelidae) was 124.03 mg (ai)L-1 which was much higher than the recommended rate (50 mg (ai)L-1) showing less toxicity toward the ladybeetle than target insect. Thus, they recommended that selective insecticide could be used in IPM program where biological agents working to reduce the pest impact (James, 2003; Cabral et al., 2008). It has been long established that pirimicarb is a selective systemic insecticide with contact, stomach and respiratory action, which is harmless and it could be applied in the IPM program against aphids. IOBC tests showed that pirimicarb is not toxic to several natural enemies, including several coccinellid species (Sterk et al., 1999). Jalali et al (2009) indicated that pirimicarb is harmless to Adalia bipunctata (Coleop.: Concinellidae). Cabral et al. (2008) showed the safety of pirimicarb to Coccinella undecimpuncata preimaginal stages and adults.
Based on LC50 values obtained in this study, third instar larvae of coccinellid beetle was 7 and 932 times more tolerant than black bean aphids to thiametoxam and pirimicarb, respectively although the methods of assays were different against two insects.
Toxicity of insecticides to different insects are vary based on different biogogical and physiological factors including reduced penetration, enhanced metabolic degradation, and altered target site (Wilkinson, 1976). Cho et al. (2002) suggested that a significant factor for the selective action of the insecticide toward larvae and adults of Harmonia axyridis than two aphid species, Aphis citricola and Myzus malisuctus, was due to differences in rates of the pesticide penetration. Indeed, genetically resistance to pesticides are less common in natural enemies than herbivorous pests due to spending less time in treated habitats and lack of pre-adaptation, generally possess lower detoxification capacity and lower genetic variability (Georghiou and Saito, 1983; Tabashnik, 1986).
In this study it was found that different esterase isoform present in the ladybeetle when B-Na was used to detect the enzyme showing the insect capacity to tolerate pesticides more than its prey.
Esterases are important detoxification enzymes involved in the resistance of many insects to organophosphates, carbamates, and pyrethroids, through gene amplification, up-regulation, coding sequence mutations, or a combination of these mechanisms (Li et al., 2007). Two main mechanisms involved in the pirimicarb resistance of Myzus persicae was AChE insensitivity caused by the Ser461Phe mutation and overexpressed E4 CbE as a supporting factor (Kown et al 2009).
Kwon et al (2009) showed that E4 CbE might provide the insect a broad non-specific resistance to certain groups of insecticides including neonicotinoids. This unusual esterase involvement in pyrethroid resistance is also well documented in Myzus persicae (Abd El-Latif and Subrahmanyam, 2010). Esterases are often found in multigene families and their amplification is responsible for enhanced degradation and sequestration of a wide range of insecticides (Oakshott et al., 1993; Zhu et al., 2011).
It is concluded that thiamethoxam and pirimicarb affect both the aphid and its predator but with different toxicity. Aphid was much more susceptible than the beetle. In enzyme assays it was found that pirimicarb does affect AChE activity as well as general esterase (Alph-NA) of the aphid whilst it does not affect AChE activity and general esterase (Alph-NA) of the beetle that is explains why the beetle is less susceptible to the pesticides. Moreover, there are four general esterase isoenzymes in the beetle whilst only one esterase band was observed in the aphid
3 Materials and methods
3.1 Test insects
A. fabae reared on broad bean plants (Vicia faba) at the constant condition in a growth chamber at 22 ± 1 °C, 70 ± 10% RH, and a photoperiod of 16:8 (L:D). Broad bean plants (Vicia faba) were planted in pots filled with sawdust and fertilized with HortiGrow (product of Hortiland, Holand) quality fertilizer with micro elements (N: K: P: Mg= 20: 0: 20: 2 percent; 1-2 kg per 1000L water) twice per week.
In addition to the tests, the aphids were used for feeding the ladybeetles. H. variegata collected from alfalfa farm of province of Alborz, Iran, maintained in Incubator, at 27 ± 1° C, 70 ± 10% RH, and a photoperiod of 16:8 (L:D).
3.2 Chemicals and Insecticides
Insecticide used in this experiment were Thiamethoxam (commercial formulation, Actara®, WG 25%, Syngenta, India) and Pirimicarb (commercial formulation, primore®, WP 50%, Golsam e Gorgan, Iran).
Acetylthiocholine iodide (ATChI), 5,5’-dithiobis-2-nitrobenzoic acid (DTNB), 1- naphthyl acetate (a-NA), b- naphthyl acetate (b-NA) and Fast Blue RR, were obtained from Sigma (www.sigmaaldrich.com). Bovine serum albumin was purchased from Bio Rad®.
3.3 Lethal toxicity bioassays
The contact method was used for H. variegata bioassay. In the experiment the third instar of larvae obtained from six hours cohort eggs, were used. Six serial dilutions of thiamethoxam (150, 354, 741, 1659, 3715, 8400 mg (ai)L-1) and pirimicarb (1600, 2754, 4786, 8317, 14454, 25600 mg (ai)L-1) were used in order to do assays against ladybeetle using topical application. Also, five serial dilutions of pirimicarb (0.25, 0.7, 1.94, 5.37, 15 mg (ai)L-1) and thiamethoxam (17.5, 43.6, 112.2, 288.4, 750 mg (ai)L-1) were used to evaluate its toxicity against the aphids in a residual assay.
Toxicity of the insecticides on the coccinellid beetle was assessed by application of one micro-liter of each solution on the larvae dorsal abdomen using micropipette. Before any treatment, the larvae were maintained at 4°C for 5 min to reduce locomotion activity during applications. For each concentration (treatment) in every six replications, 79 and 74 insects for thiamethoxam and pirimicarb, respectively were used. Treated insects in groups of 4 or 5 individuals, were put on Petri dishes (60 mm in diameter´10mm in height) and sufficient Aphis fabae (in all stages) were put in the Petri dish in order to provide food for them. Mortality was assessed 24 h post-treatment.
A residual contact method was used for A. fabae bioassays based on Kwon et al., 2009 procedures. For each treatment 120 insects were used in three replications.
Insecticide solutions were prepared by dissolving appropriate amounts of insecticide formulations in distilled water. Broad bean leaves from plants in the same age, were cut and soaked in serially diluted insecticide solutions for 30 s and dried under hoods for 60 min. The leaf disk was placed over agar-agar 1.2% in a petri dish (60 mm in diameter´10mm in height), and 20 wingless female adult aphids were transferred onto the leaf disk. Mortality was evaluated at 24 h post-treatment. Aphids showing uncoordinated movement when prodded were counted as dead.
3.4 Sublethal assays
After determination of LCs, the insects were exposed to sublethal concentrations of both insecticides. Coccinellid larvae were exposed to 652.13 and 2740 mg (ai)L-1 of pirimicarb and 48.21 and 788.55 mg (ai)L-1 of thiamethoxam. The aphids were treated with concentrations of 0.93, 1.84, and 2.94 mg (ai)L-1 of pirimicarb and 13.85, 48.07 and 113.85 mg (ai)L-1 of thiamethoxam. After 24h, the survival individuals were collected and used for subsequent experiments.
3.4.1 Assay of AChE activity
AChE assays was done basically according to Ellman et al. (1960) and Zhu and Clark (1994). Briefly, for AChE, 60 aphids and 5 coccinellid larvae from each treatment were homogenized in 0.1 M sodium phosphate buffer, pH 7.5 containing 0.1% (v/v) Triton X-100. The homogenates were centrifuged for 30 min at 15,000g and the supernatants were used for the enzyme assay.
To do assay, 50 µl crude enzyme and 150 µl of solution containing AChI (1.5 mM) and DTNB (1 mM) in 0.1 M sodium phosphate buffer, pH 7.5 was mixed and the activity was measured at 405 nm in a continuance manner during 10 min. All experiments were conducted in three replicates.
3.4.2 Assay of general esterase activity
For general esterase assay, 30 live wingless aphids and 5 live coccinellid larvae from each treatment were collected and homogenized in 0.04 M sodium phosphate buffer, pH 7.0 respectively. The homogenates were centrifuged for 20 min at 10,000g and the supernatants were collected and used for enzyme assay (Brogdon and Dickinson, 1983).
To determine esterase activity, 90 µl of solution (20 µl crude enzyme and 70 µl BPS, 0.04 M, pH7.0) was incubated with 90 µl substrate (56 mg a-NA or b-NA in 10 ml acetone which further dissolved in 90 ml BPS) for 10 min at 30°C. The reaction was terminated by addition of 0.1% Fast blue RR salt solution. The absorbance was measured at 450 nm continuously over 10 minutes using a microplate reader (Model 550, Bio-Rad, Hercules, CA).
3.4.3 Polyacrylamide gel electrophoresis (PAGE)
Non-denaturing polyacrylamide gel electrophoresis (PAGE) was performed using the Laemmli (1970) buffer system without sodium dodecil sulfate (SDS) and a gel system consisting of a 4% (w/v) stacking gel and a 7.5% (w/v) separating gel based on.
Carboxylesterases were electrophoresed with 7.5% tris-glycine (pH 8.3) as a running buffer, according to the methods of Kono and Tomita (1992). After separation, the gel was incubated in phosphate buffer solution, 0.1 M, pH 6.0, containing 30 mM a- and/or b -NA as a substrate and 0.15 % fast blue RR salt at room temperature for 30 min.
3.5 Protein assay
Protein contents of the enzyme homogenate were determined according to the method of Bradford (1976) using bovine serum albumin as standard. The measurement was performed with the wavelength of 630 nm.
3.6 Statistical analysis
In the toxicity test, concentration-mortality regression for the larvae was evaluated using probit analysis (Polo-PC Probit and Logit analysis; LeOra Software 1997). Differences in toxicity were considered significant when 95% fiducial Limit (FL) did not overlap (Adams, Hall, Hoy, 1990).
Mean and standard deviation values were determined for all the biochemical parameters. The data were analyzed employing analysis of one-way variance (ANOVA). Fisher’s least significant difference (LSD) multiple comparisons were then used to separate the means among the treatments for each biochemical parameter. All the statistical analyses were performed using the General Linear Model (GLM) procedure of SAS (version 9.1.3) software. P-values below 0.05 were regarded as significant.
Abd El-Latif AO., and Subrahmanyam B., 2010, Pyrethroid resistance and esterase activity in three strains of the cotton bollworm, Helicoverpa armigera (Hübner), Pestic. Biochem. Physiol., 96: 155-159
https://doi.org/10.1016/j.pestbp.2009.11.004
Agarwala R.K., and Dixon A.F.G., 1992, Labratory study of cannibalism and interspesific predation in ladybirds, Ecol. Entomol., 17 (3): 303-330
https://doi.org/10.1111/j.1365-2311.1992.tb01062.x
Arocca A., Fanti P., Molinaro A., M.F. Mattia, and Battaglia D., 2011, Aphid performance on Vicia faba and two southern Italy Phaseolus vulgaris landraces, Bull. Insectol., 64 (1): 101-106
Badiou A., Meled M., and Belzunces LP., 2008, Honeybee Apis mellifera acetylcholinesterase-A biomarker to detect deltamethrin exposure. Ecotoxicology and Environmental Safety, 69: 246-253
https://doi.org/10.1016/j.ecoenv.2006.11.020
PMid:17215041
Baffi M.A., Pereira C.D., de Souza G.R.L., Bonetti A.M., Ceron C.R. and Gourlart L.R., 2005, Esterase profile in a pyrethroid-resistant Brazilian strain of the cattle tick Boophilus microplus (Acari: Ixodidae), Genet. Mol. Biol., 28(4): 749-753
Bandyopadhyay, R., 1982, Inhibition of acetylcholine esterase by permethrin and its reversion by acetylthiocholine. Indian J. Exp. Biol., 20: 488-491
Bass C., Puinean A.M., Zimmer C. T., Denholm I., Foster S.P., Gutbrod O., Nauen R., Slater R., Field L.M., and Williamson M.S., 2014, The evolution of insecticide resistance in the peach potato aphid, Myzus persicae, Insect Biochem. Mol. Biol., 51: 41-51
https://doi.org/10.1016/j.ibmb.2014.05.003
PMid:24855024
Bergmann F., Segal R., and Rimon S., 1957, A new type of esterase in hog-kidney extract, Biochem. J., 67: 481-486
https://doi.org/10.1042/bj0670481
PMid:13479408 PMCid:PMC1200182
Booth L.H., Wratten S.D. And Kehrli P., 2007, Effects of reduced rates of two insecticides on enzyme activity and mortality of an aphid and its lacewing predator, J. Econ. Entomol., 100: 11-19
https://doi.org/10.1093/jee/100.1.11
PMid:17370803
Bradford M.M., 1976, A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding, Anal. Biochem., 72: 248-254
https://doi.org/10.1016/0003-2697(76)90527-3
Brogdon W.G. and Dickinson C.M., 1983, A microassay system for measuring esterase activity and protein concentration in small samples and in high-pressure liquid chromatography elute fraction, Anal. Biochem., 131: 499-503
https://doi.org/10.1016/0003-2697(83)90204-X
Cabral S., Garcia P., and Soares A.O., 2008. Effects of pirimicarb, buprofezin and pymetrozine on survival, development and reproduction of Coccinella undecimpunctata (Coleoptera: Coccinellidae), Biocontrol Sci. Technol., 18: 307-318
https://doi.org/10.1080/09583150801902072
Chao S.L., Dennehy T.J., and Casida J.E., 1997, Whitefly (Hemiptera: Aleyrodidae) binding site for imidacloprid and related insecticides: A putative nicotinic acetylcholine receptor. J Econ. Entomol., 90: 879–882
https://doi.org/10.1093/jee/90.4.879
PMid:9260539
Cho J.R., Kinnt Y.J., Kim H.S. and Yoo J.K., 2002, Some Biochemical Evidence on the Selective Insecticide Toxicity between the Two Aphids, Aphis citricola and Myzus rnalisuctus (Homoptera: Phididae), and Their Predator, Harmonia axyridis (Coleoptera: Coccinellidae). J. Asia-Pacific Entomol., 5 (1): 49-53
https://doi.org/10.1016/S1226-8615(08)60132-6
Dewar A.M, Dewar A.J.G., Haylock L.A., Foster S.P. and Williamson M.S., 2014, Alternative insecticides to control cereal aphids, Sitobion avenae, that are resistant to pyrethroids, Proceedings Crop Protection in Northern Britain, 131-136
Diehl E., Sereda E., Wolters V. and Birkhofer K., 2013, Effects of predator specialization, host plant and climate on biological control of aphids by natural enemies: a meta-analysis, J. Appl. Ecol., 50: 262–270
https://doi.org/10.1111/1365-2664.12032
Ellman G.L., Courtney D.K., Andres V. and Featherstone RM., 1961, A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol., 7: 88-95
https://doi.org/10.1016/0006-2952(61)90145-9
Franzmann BA., 2002. Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae), a predacious ladybird new in Australia, Aust. J. Entomol., 41: 375-377
https://doi.org/10.1046/j.1440-6055.2002.00318.x
Gardiner M.M., Allee L.L., Brown P.M.J., Losey J.E., Roy H.E. and Smyth R.R., 2012, Lessons from lady beetles: accuracy of monitoring data from US and UK citizen-science programs. Front. Ecol. Environ., 10 (9): 471-476
https://doi.org/10.1890/110185
Georghiou, G.P., and Saito T., eds., 1983, Pest Resistance to Pesticides, Plenum, New York, p. 669.
https://doi.org/10.1007/978-1-4684-4466-7
Gordon RD., 1987, The first North American records of Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). J. N Y Entomol. Soc., 95: 307-309
Herath P.R.J., Hemingway J., Weerasinghe I.S., and Jayawardena K.G.I., 1987, The detection and characterization of malathion resistance in field populations of Anopheles culicifacies B in Sri Lanka, Pestic. Biochem. Physiol., 29: 157-162
https://doi.org/10.1016/0048-3575(87)90074-5
Hippa H., Kepeken S.D., and Laine T., 1978, On the feeding biology of Coccinella hieroglyphica L. (Coleoptera, Coccinellidae), Kevo-subaretitic Ras. Station 14 (2): 18-20
Honek A., Martinkova Z., Kindlmann P., Ameixa O.M.C.C., and Dixon A.F.G., 2014, Long-term trends in the composition of aphidophagous coccinellid communities in Central Europe, Insect Conserv. Diver., 7: 55–63
https://doi.org/10.1016/0048-3575(87)90074-5
Hughes J., Reay G. and Watson J., 2014, Insecticide use on scottish oilseed rape crops: historical use patterns and pest control options in the absence of neonicotinoid seed treatments, Proceedings Crop Protection in Northern Britain, 21-26
Jalali MA., Van Leeuwen T., Tirry L. and De Clercq P., 2009, Toxicity of selected insecticides to the two-spot ladybird Adalia bipunctata. Phytoparasitica, 37: 323-326
https://doi.org/10.1007/s12600-009-0051-6
Jepson, P.C. ed., 1989, Pesticides and Non-Target Invertebrates. Intercept, Wimborne, Dorset, pp. 95-128.
Karunaratne S.H.P.P., Hemingway J., Weerasinghe I.S., Jayawardena K.G.I., Dassanayaka V. and Vaughan A., 1995, Kinetic and molecular differences in the amplified and nonamplified esterases from insecticide-resistant and susceptible Culex quinquefasciatus mosquitoes, J. Biol. Chem., 270: 31124-31128
https://doi.org/10.1007/s12600-009-0051-6
Karunaratne S.H.P.P., Weerakoon KC., Nugaliyadda L. and Manuweera GK., 2007,Susceptibility of rice insect pests and their natural enemies to commonly used insecticides J. Natn. Sci. Foundation Sri Lanka, 35(2): 97-102.
https://doi.org/10.4038/jnsfsr.v35i2.3673
Kennedy JS., Day MF. and Eastop VF., 1962. A Conspectus of Aphids as Vectors of Plant Viruses. Commonwealth Inst. Ent., London, 114 pp.
Kono, Y. and Tomita T., 1992, Characteristics of highly active carboxylesterases in insecticide-resistant Culex pipiens quinquefasciatus, Jpn. J. Sanit. Zool. 43: 297–305
https://doi.org/10.7601/mez.43.297
Kontodimas DC., Stathas GJ., 2005, Phenology, fecundity and life table parameters of the predator Hippodamia variegata reared on Dysaphis crataegi. Biocontrol, 50: 223-233
https://doi.org/10.1007/s10526-004-0455-7
Kring T.J., Gilstrap F.E., and Michels G.I. 1985, Role of indigenous coccinellid in regulating green bugs on Texas grain sorghum, J. Econ. Entomol., 78 (1): 269-273
https://doi.org/10.1093/jee/78.1.269
Kumral N. A., Gencer N. S., Susurluk H. and C. Yalcin, 2011, A comparative evaluation of the susceptibility to insecticides and detoxifying enzyme activities in Stethorus gilvifrons (Coleoptera: Coccinellidae) and Panonychus ulmi (Acarina: Tetranychidae), International Journal of Acarology, 37: 255-268
https://doi.org/10.1080/01647954.2010.514289
Kwon D.H., Choi B.R., Lee S.W., Clark J.M. and Lee S.H., 2009. Characterization of carboxylesterase-mediated pirimicarb resistance in Myzus persicae, Pestic. Biochem. Physiol., 93: 120-126
https://doi.org/10.1016/j.pestbp.2008.12.001
Laemmli U.K., 1970, Cleavage of structural protein during assembly of the head of bacteriophage T4. Nature, 227: 680-685
https://doi.org/10.1038/227680a0
PMid:5432063
Lima D.B. , Monteiro V.B. , Guedes R.N.C. , Siqueira H.A.A. , Pallini A., and Gondim M.G.C., Jr., 2013, Acaricide toxicity and synergism of fenpyroximate to the coconut mite predator Neoseiulus baraki , BioControl, 58(5): 595-605
https://doi.org/10.1007/s10526-013-9520-4
Li X.C., Schuler M.A., and Berenbaum M.R., 2007, Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics, Annu. Rev. Entomol. 52: 231-253
https://doi.org/10.1146/annurev.ento.51.110104.151104
PMid:16925478
McCarthy, J.F., and Shugart L.R., 1990. Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, FL.
Nauen R, Koob B, and Elbert A., 1998, Antifeedant effects of sublethal dosages of imidacloprid on Bemisia tabaci. Entomol. Exp. Appl., 88: 287–293
https://doi.org/10.1046/j.1570-7458.1998.00373.x
Rahmani S. and Bandani A.R., 2013, Sublethal concentrations of thiamethoxam adversely affect life table parameters of the aphid predator, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Crop Prot., 54: 168-175
https://doi.org/10.1016/j.cropro.2013.08.002
Raboudi F., Benmoussa A., Makni H., and Marrakchi M. 2002, Serological detection of plant viruses in their aphid vectors and host plants in Tunisia Bull. OEPP/EPPO Bull., 32 (4): 495-498
https://doi.org/10.1046/j.1365-2338.2002.00596.x
Rao, GV. and Rao, KS., 1995, Modulation in acetylcholinesterase of rat brain by pyrethroids in vivo and an in vitro kinetic study, J. Neurochem. 65: 2259-2266
https://doi.org/10.1046/j.1471-4159.1995.65052259.x
PMid:7595515
Sharma D.R. and Lal O.P., 2002, Bio-efficacy of thiamethoxam in comparison to recommended insecticides against leafhopper and white fly of brinjal (Solanummelongena L.), J. Entomol. Res., 26: 257–262
Stark JD. and Banks JE., 2003, Population-level effects of pesticides and other toxicants on arthropods, Annu. Rev. Entomol., 48: 505-519
https://doi.org/10.1146/annurev.ento.48.091801.112621
PMid:12221038
Sterk, G., Hassan, S.A., Baillod, M., Bakker, F., Bigler, F., Blu¨mel, S., Bogenschu¨ tz, H., Boller, E., Bromand, B., Brun, J., Calis, J.N.M., Coremans-Pelseneer, J., Duso, C., Garrido, A., Grove, A., Heimbach, U., Hokkanen, H., Jacas, J., Lewis, G., Moreth, L., Polgar, L., Roversti, L., Samsoe-Pettersen, L., Sauphanor, B., Schaub, L., Sta¨ubli, A., Tust, J.J., Vainio, A., Van De Veire, M., Viggiani, G., Vin˜uela, E., and Vogt, H., 1999. ‘‘Results of the Seventh Joint Pesticide Testing Programme Carried Out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’, BioControl, 44: 99-117
https://doi.org/10.1023/A:1009959009802
Syngenta, 2004. Cata´logo, Syngenta Crop Protection, Syngenta, Lisboa, Portugal.
Van Emden H.F., and Harrington R., eds., 2007. Aphids as Crop Pests, pp. 717 CAB International, Trowbridge.
https://doi.org/10.1079/9780851998190.0000
PMid:17988728
Volk W., and Stechmann D.H., 1998, Parasitism of the black bean aphid (Aphis fabae) by Lysiphlebus fabarum (Hymenoptera, Aphidiidae): the influence of host plant and habitat, J. Appl. Entomol., 122: 201-206
https://doi.org/10.1111/j.1439-0418.1998.tb01484.x
Yang C.J., Yuan F., Hua B.Z., Sun J.J., Lei YX. and Zhao S.F., 1997. Spatial distribution patterns and sampling techniques of Hippodarnia variegata (Goeze) on the tobacco fields in northern Shaanx, Entomol. Knowl., 34: 283-288.
Walker C.H. and Mackness M.I., 1983. Esterases: problems of identification and classification, Biochem. Pharmacol. 32: 3265-3269
https://doi.org/10.1016/0006-2952(83)90349-0
Wu G. and Miyata T., 2005, Susceptibilities to methamidophos and enzymatic characteristics in 18 species of pest insects and their natural enemies in crucifer vegetable crops, Pestic. Biochem. Physiol., 82: 79-93
https://doi.org/10.1016/j.pestbp.2005.01.001
Wu X.H., Zhou X.R. and Pang B.P., 2010, Influence of five host plants of Aphis gossypii Glover on some population parameters of Hippodamia variegata (Goeze). J. Pest Sci., 83: 77-83
https://doi.org/10.1007/s10340-009-0272-y
Youn Y.N., Seo MJ., Shin J.G., Jang C. and Yu YM., 2003, Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae), Biol. Control, 28: 164-170
https://doi.org/10.1016/S1049-9644(03)00098-7
Yu S.J., 1987, Biochemical defense capacity in the spined soldier bug (Podisus maculiventris) and its lepidopterous prey, Pestic. Biochem. Physiol., 28: 216-223
https://doi.org/10.1016/0048-3575(87)90020-4
Yu, S.J., 1988, Selectivity of insecticides to the spined soldier bug (Heteroptera: Pentatomidae) and its lepidopterous prey, J. Econ. Entomol., 81: 119-122
https://doi.org/10.1093/jee/81.1.119
Zhu YC., West S., Snodgrass G. and Luttrell R., 2011, Variability in resistance-related enzyme activities in field populations of the tarnished plant bug, Lygus lineolaris, Pestic. Biochem. Physiol. 99 (3): 265–273
https://doi.org/10.1016/j.pestbp.2011.01.005
Zhu KY., Clark JM., 1994, Purification and characterization of acetylcholinesterase from the Colorado potato beetle, Leptinotaras decemlineata (Say), Insect Biochem. Molec. Biol., 24: 453-461
. PDF(1179KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Shima Rahmani
. Ali R. Bandani
Related articles
. Carboxylesterase
. Acetylcholinesterase
. Selective insecticides
. Hippodamia variegata
. Aphis fabae
Tools
. Email to a friend
. Post a comment
.png)
.png)