 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2024, Vol. 14, No. 1 doi: 10.5376/jmr.2024.14.0003
Received: 27 Mar., 2024 Accepted: 01 Apr., 2024 Published: 05 Apr., 2024
Xuan J., 2024, Comparative analysis of dengue and zika virus transmissibility in primates, Journal of Mosquito Research, 14(1): 18-25 (doi: 10.5376/jmr.2024.14.0003)
The paper "Trade-offs shaping transmission of sylvatic dengue and Zika viruses in monkey hosts" authored by Kathryn A. Hanley, Hélène Cecilia, Sasha R. Azar, et al., was published in Nature Communications on March 27, 2024. The authors are affiliated with Department of Biology, New Mexico State University, Department of Pathology, University of Texas Medical School, among other institutions. This study explores the factors affecting the transmission of Dengue (DENV) and Zika (ZIKV) viruses in monkey hosts. By experimentally infecting Asian ancestral host species (rhesus monkeys) and a New World host species (squirrel monkeys), the study investigated viral replication, innate and adaptive immune responses (natural killer cells and neutralizing antibodies), and transmission to mosquitoes. Results show that ZIKV reached high titers in both hosts and translated to high mosquito transmission rates; in contrast, DENV-2 had lower replication levels, with transmission occurring only when serum viral titers were below or near the detection limit. The study reveals the immune-mediated trade-offs between the duration and intensity of viral replication.
The paper "Trade-offs shaping transmission of sylvatic dengue and Zika viruses in monkey hosts" authored by Kathryn A. Hanley, Hélène Cecilia, Sasha R. Azar, et al., was published in Nature Communications on March 27, 2024. The authors are affiliated with Department of Biology, New Mexico State University, Department of Pathology, University of Texas Medical School, among other institutions. This study explores the factors affecting the transmission of Dengue (DENV) and Zika (ZIKV) viruses in monkey hosts. By experimentally infecting Asian ancestral host species (rhesus monkeys) and a New World host species (squirrel monkeys), the study investigated viral replication, innate and adaptive immune responses (natural killer cells and neutralizing antibodies), and transmission to mosquitoes. Results show that ZIKV reached high titers in both hosts and translated to high mosquito transmission rates; in contrast, DENV-2 had lower replication levels, with transmission occurring only when serum viral titers were below or near the detection limit. The study reveals the immune-mediated trade-offs between the duration and intensity of viral replication.
1 Experimental Data Analysis
This study investigates the replication and transmission capabilities of DENV-2 and ZIKV in hosts, finding that ZIKV can achieve high replication levels in hosts, closely associated with higher mosquito transmission rates. In contrast, DENV-2 has lower replication levels in hosts. Additionally, the study points out that the activity of natural killer (NK) cells is related to ZIKV replication levels and DENV-2 antibody levels, highlighting the role of host immune responses in virus transmission. The experiments also detailed the animal models, virus strains, and mosquito species used, ensuring the study's replicability and providing a reliable experimental framework for future research. These findings offer important insights into understanding virus transmission mechanisms and developing prevention and control strategies.
Figure 1 presents the experimental design and subsequent sample collection process for infections with Dengue virus serotype 2 (DENV-2) and Zika virus (ZIKV) in rhesus monkeys and squirrel monkeys. The experiment monitored not only viremia and natural killer (NK) cell counts but also measured neutralizing antibodies via the Plaque Reduction Neutralization Test (PRNT), along with body temperature recorded by implanted sensors in the monkeys and body weight measured on specific days. The infection and monitoring process for both rhesus and squirrel monkeys were largely the same, with a few differences: for instance, the number of uninfected Aedes mosquitoes used for monitoring transmission was 10 for the DENV-2 infected rhesus monkeys and control groups, increased to 15 for the ZIKV infected rhesus monkeys and all squirrel monkeys; fecal collection for occult blood screening was conducted daily in rhesus monkeys but not in squirrel monkeys due to pair housing; additionally, at the end of the experiment, squirrel monkeys were euthanized and dissected, while rhesus monkeys were not. These meticulously documented experimental procedures provide strict experimental conditions for further data analysis.
 Figure 1 Figure 1 Overview of experimental infections of cynomolgus macaques and squirrel monkeys with dengue virus serotype 2 (DENV-2, in green) and Zika virus (ZIKV, in blue) and subsequent sampling |
Table 1 shows rhesus monkeys being assigned to low-dose (1 infected mosquito) or high-dose (10 infected mosquitoes) Dengue virus (DENV) experimental groups, or a high-dose (15 infected mosquitoes) Zika virus (ZIKV) experimental group, along with a control group (10 uninfected mosquitoes). The results indicate that the presence days and peak titers of the virus in monkeys varied with different doses of viral infection, with the high-dose ZIKV infection group generally having a higher proportion of virus transmission to mosquitoes than the DENV groups. Moreover, the data in the table reflect the neutralizing efficacy of the neutralizing antibodies produced in monkeys, with some animals not detecting the virus on specific days, suggesting individual differences in the host's immune response to the virus. These data provide experimental evidence for understanding the impact of different viral doses on the host and their transmission capability to mosquitoes.
.png) Table 1 Assignment of each cynomolgus macaque to low dose (1 infected mosquito) or high dose (10 infected mosquitoes) of DENV, or high dose (15 infected mosquitoes) of ZIKV, or control (10 uninfected mosquitoes) treatments and subsequent viremia, transmission to mosquitoes, and neutralizing antibody response |
Figure 2 provides a comprehensive analysis of the effects of treatment with Dengue virus serotype 2 (DENV-2) and Zika virus (ZIKV) on rhesus monkeys and squirrel monkeys. The average peak titers and their 80% plaque reduction neutralization titers (PRNT80) for the rhesus monkeys show that ZIKV has stronger replicative ability than DENV-2. Analysis of squirrel monkeys also revealed similar results, with higher average peak titers for ZIKV and lower average PRNT80 against ZIKV, suggesting that ZIKV may induce a stronger immune response. Moreover, early natural killer (NK) cell percentages were negatively correlated with PRNT80 values, particularly in monkeys infected with DENV-2, and early NK cell percentages were also negatively correlated with peak viremia and duration of viremia for ZIKV, revealing the role of host immune cells in the clearance of the virus.
 Figure 2 Effect of treatments on peak virus titer, 80% plaque reduction neutralization titer (PRNT80), and natural killer (NK) cells, and relationships among them, for dengue virus serotype 2 (DENV-2, in green) and Zika virus (ZIKV, in blue); values for individual animals shown as points |
Table 2 summarizes the results following virus delivery to squirrel monkeys via cartons of 15 mosquitoes, including viremia, virus transmission to mosquitoes, and neutralizing antibody response. The table records individual and control group data for Dengue virus (DENV) and Zika virus (ZIKV), along with corresponding experimental data. Results indicate that squirrel monkeys treated with ZIKV exhibited higher peak viremia and mosquito infection rates. For instance, squirrel monkeys treated with ZIKV showed a viremia duration of 3 to 5 days and higher mosquito transmission rates, reflecting ZIKV's higher transmission potential. Moreover, PRNT80 results suggest that the presence of the virus in the host does not always lead to effective mosquito infection, reflecting the complex relationship between viral exposure in the host and immune protection.
.png) Table 2 Virus delivered to squirrel monkeys via cartons of 15 mosquitoes and subsequent viremia, transmission to mosquitoes, and neutralizing antibody response |
Figure 3 illustrates the replication and transmission of wild-type Dengue virus serotype 2 (DENV-2) in ancestral hosts (rhesus monkeys) and new hosts (squirrel monkeys). In both of these primate species, DENV-2 replication levels were low, and transmission to Aedes albopictus mosquitoes occurred infrequently. When viremia was not detected in rhesus and squirrel monkeys, their data points for mosquito infection were placed below the limit of detection (LOD) at an arbitrary position for ease of observation. Viremia was monitored by direct titration of sera and also by titration after one passage of the serum; transmission monitoring involved allowing 10 to 15 uninfected mosquitoes to feed on each monkey. Typically, the number of mosquitoes that fed was less than the total number of mosquitoes prepared.
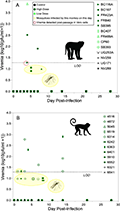 Figure 3 Replication and transmission of sylvatic dengue virus serotype 2 (DENV-2) is similar in native (cynomolgus macaque) and novel (squirrel monkey) hosts |
Figure 4 reveals the relationship between viral titers in non-human primates and the probability of virus transmission to mosquitoes. For wild-type Dengue virus serotype 2 (DENV-2), transmission to mosquitoes occurred only when the viral titers in serum were below or near the detection limit, while for wild-type Zika virus (ZIKV), the viral titers in serum positively correlated with transmission to mosquitoes. The curve fitted by a generalized additive model shows that the probability of ZIKV transmission to mosquitoes increases with higher viral titers, whereas the transmission probability for DENV-2 peaks at lower viral titers. This finding highlights the differences in replication and transmission capabilities among different viruses within host bodies.
 Figure 4 Transmission of DENV-2 (in green) from non-human primates to mosquitoes occurred only when serum viremia was below or near the limit of detection, while ZIKV (in blue) transmission was positively associated with serum viremia |
Figure 5 compares the replication and transmission efficiency of wild-type Zika virus (ZIKV) in its ancestral hosts (rhesus monkeys) and new hosts (squirrel monkeys). In both hosts, ZIKV demonstrated robust replication capabilities and effectively transmitted to Aedes albopictus mosquitoes. The blue line represents the average viremia levels, the blue band represents the standard error, and the black dots represent individual viral viremia data for each monkey. The bar charts show the average percentage of mosquitoes infected by each monkey, with error bars representing standard error. In the illustrations for rhesus monkeys, the dynamics of viral replication and transmission within the viremia window period are revealed. Transmission was monitored by feeding 15 uninfected mosquitoes to each monkey, with typically fewer than 15 mosquitoes feeding.
 Figure 5 Replication and transmission of sylvatic Zika virus (ZIKV) is similar in native (cynomologus macaque) and novel (squirrel monkey) hosts |
Figure 6 demonstrates scenarios where the novel host exhibits higher efficiency in transmitting Zika virus (ZIKV) to mosquitoes compared to the ancestral host. In Chart A, dark blue represents squirrel monkeys and light blue represents rhesus monkeys, with the size of the data points proportional to the number of mosquitoes tested, showing the relationship between serum viral titers and the probability of ZIKV transmission to the legs of Aedes mosquitoes. Charts B to E compare the ZIKV data (blue lines) of these two primate hosts with different serotypes of Dengue virus (DENV) transmitted to the legs of Aedes aegypti mosquitoes from human hosts. The shown curves are the best fits, with the shaded area representing the 95% confidence interval of the fitting process. These model analyses reveal that the novel host, squirrel monkeys, have a stronger ability to transmit ZIKV to mosquitoes compared to human hosts, providing valuable insights into how the virus transmits between different hosts.
 Figure 6 Novel hosts transmitted ZIKV to mosquitoes more efficiently than native hosts |
2 Analysis of Research Findings
This study provides new insights into the replication of Dengue virus (DENV-2) and Zika virus (ZIKV) in monkey hosts and their relationship with mosquito transmission. It was found that ZIKV can achieve higher levels of replication in monkey hosts, closely associated with its high transmission rate in mosquitoes, suggesting ZIKV's strong potential for transmission, which helps establish stable transmission cycles in new host populations. In contrast, the lower replication efficiency of DENV-2 may limit its ability to spread through mosquitoes in the wild. Understanding these differences in viral behavior, particularly in terms of replication and transmission patterns across different hosts, is crucial for understanding how viruses adapt and spread in various ecological settings. The findings of this study deepen our understanding of virus transmission dynamics and provide a scientific basis for developing effective disease prevention and control strategies, highlighting the importance of studying the interactions between viruses, hosts, and vectors.
3 Evaluation of the Research
This study, through its meticulously designed experiments, detailed data collection, and rigorous analytical methods, demonstrates the high degree of precision in scientific research. It delves into the transmission mechanisms and immune responses of Dengue virus (DENV) and Zika virus (ZIKV) in non-human primate hosts, providing crucial insights into how these viruses replicate within hosts and their impact on mosquito transmission capabilities. The research team maintained high accuracy throughout the experimental process and detailed the experimental steps and analytical methods in the report. This not only ensured the credibility and transparency of the research findings but also enhanced the study's replicability. This methodological rigor and transparency serve as a valuable example and reference for future research in virology and its intersecting fields, helping to advance the scientific community's understanding of the complex relationship between virus transmission and host immune responses, while laying a solid foundation for developing effective prevention measures and treatment methods.
4 Conclusion
By precisely mimicking the process of monkeys being infected with Dengue virus (DENV) and Zika virus (ZIKV) through mosquito bites, this study has revealed complex and subtle interactions between virus transmission and host immune responses. These findings not only enhance our understanding of how these two viruses replicate within different hosts and their mechanisms of transmission but also hold significant implications for the development of preventative measures and treatment strategies against these viruses. Furthermore, the results underscore the importance of understanding how viruses transmit between natural hosts and humans in the field of public health, providing a scientific basis for formulating effective disease control and prevention policies. This research has broad application prospects and profound impacts.
5 Access the Full Text
Hanley, K.A., Cecilia, H., Azar, S.R. et al. Trade-offs shaping transmission of sylvatic dengue and Zika viruses in monkey hosts. Nat Commun 15, 2682 (2024). https://doi.org/10.1038/s41467-024-46810-x
Acknowledgments
I sincerely appreciate the valuable opportunity provided by Nature Communications to share the latest academic progress on the transmission and immune responses of Dengue virus (DENV) and Zika virus (ZIKV) in non-human primate hosts through writing this scientific review with a wide readership. This platform serves not only as a showcase for research findings but also as an important conduit for promoting academic exchange and enhancing public awareness of the significance of scientific research. Through this review, we hope to spark further interest among researchers in this field, while also raising public awareness about the prevention and control of these significant viral diseases.
. PDF(574KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jia Xuan
Related articles
. Dengue
. Zika Virus
Tools
. Email to a friend
. Post a comment