Reseach Report
Pupicidal Activity of Ethanol and Water Extracts of Phytolacca dodecandra (L’ Herit) on Anopheles gambiae (Diptera Culicidae) Pupae 
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2017, Vol. 7, No. 13 doi: 10.5376/jmr.2017.07.0013
Received: 19 Jun., 2017 Accepted: 18 Jul., 2017 Published: 28 Jul., 2017
Yugi J.O., Kiplimo J.J., and Misire C., 2017, Pupicidal activity of ethanol and water extracts of Phytolacca dodecandra (L’ Herit) on Anopheles gambiae (Diptera: Culicidae) pupae, 7(13): 104-110 (doi: 10.5376/jmr.2017.07.0013)
Pupae are an integral part of Anopheles gambiae life history and a tool that effectively targets them is likely to impact on malaria vector density. In this study we evaluated pupicidal activities of crude ethanol and water extracts of Phytolacca dodecandra (L’ Herit) on An. gambiae (Diptera: Culicidae) under laboratory condition. Individual early pupae of An. gambiae mosquitoes were exposed to concentrations of 40, 20, 10, 5, 2.5 mgs/100 ml of mature green fruits and leaves of the shoot and midsection of P. dodecandra in containers measuring 6 cm top × 5.7 cm bottom × 3.5 cm height. P. dodecandra was sourced from Eldoret (highland) and Nyando (lowland). Extraction of P. dodecandra bioactives was done using 80% ethanol and distilled water. Extracts of Neem leaves and untreated rain water were used as positive and negative controls respectively. WHO, 2005 mortality threshold of >80% was used to determine the effectiveness of the extracts as pupicide 24 hours post exposure. Mortality of exposed pupae was dose dependent. Concentrations of 20 mgs/100 mls and higher and 40 mgs/ 100mls of mature green fruits from Nyando and Eldoret met the mortality threshold respectively. Extracts of P. dodecandra from Nyando was more potent than that from Eldoret as was water compared to ethanol extracts. Positive (Neem) control killed < 80% of exposed pupae. It was concluded that crude extracts of P. dodecandra are potent as pupicide against An. gambiae mosquitoes and is a likely alternative to synthetic insecticides against An. gambiae in future.
Background
Mosquitoes are responsible for lymphatic filariasis (Culex quinquefasciatus Say), yellow fever, dengue (Aedes aegypti Linn.) and malaria (Anopheles gambiae Giles) (Ndione et al., 2013). These infections are debilitating and deprive the sufferers the beauty of enjoying life and being useful to themselves, their families and their countries. Synthetic chemical insecticides are the tools of choice to control the vectors and prevent the mosquito transmitted infections (Brattsten and Hamilton, 2012). However, because of their indiscriminate use, mosquitoes have developed resistance, rendering the management strategy less effective. Moreover majority of the synthetic chemicals have undesirable effects on non-target organisms and even foster environmental and human health concerns (Yang et al., 2002). Because of these unfortunate effects, recent search for potent malaria vector control methods have shifted to botanicals that have been found to be quantitatively and qualitatively rich in volatile constituents (Choochote et al., 2005; Praveen et al., 2012). The extracts are also biodegradable and nontoxic to the environment (Choochote et al., 2005; Krishnappa et al., 2013; Elumalai et al., 2013).
Additionally, plant extracts have demonstrated toxicity against mosquito eggs, larvae, pupae and adults (Elumalai et al., 2012a; Elumalai et al., 2012b; Gokulakrishnan et al., 2012; Ramar et al., 2012; Ramar et al., 2014; Balu et al., 2015; Pravin et al., 2015). They also have demonstrable repellency (Pitasawat et al., 2003; Omolo et al., 2004; Traboulsi et al., 2005), and oviposition deterrents (Cavalcanti et al., 2004; Ansari et al., 2005) effects. Indeed plant products had been envisioned as possible replacement of synthetic chemicals for the management of mosquito immature (Anupam et al., 2012).
Phytolacca dodecandra plant extracts has proven effective not only against immature of filarial vector Cx. guinquefasciatus Say (Diptera: Culicidae) (Misganaw et al., 2012) but also to An. gambiae larvae (Yugi et al., 2015) and adults (Yugi et al., 2016). Reports however, on its effectiveness as larvicide has largely been on the different larval stages (Larval instars 1 to 4) without a mention of the non-feeding stage, the pupae. This study was therefore designed to test and report on the effects of crude ethanol and water extracts of P. dodecandra parts on pupae of An. gambiae in the laboratory. This was to provide information on toxicity of P. dodecandra extracts on this crucial link to the An. gambiae life history.
1 Results
This experiment was conducted for a period of three weeks using 3,690 An. gambiae pupae. It was observed that water extracts of mature green fruits of P. dodecandra were more toxic compared to ethanol extracts of the same part. Extracts of P. dodecandra from the lowlands (Nyando) was more potent than that from the highlands (Eldoret). It was also found that the activity of P. dodecandra extracts was dose dependent with mean percent mortality increasing with increased concentrations irrespective of part of P. dodecandra or solvent used. Only concentration of 40 mgs/100 mls from mature green fruits sourced from the highlands (Eldoret) killed > 80% of exposed pupae irrespective of solvent used (Table 1). Concentrations of 20 mgs/100 mls and higher from mature green fruits sourced from the lowlands (Nyando) killed > 80% of exposed pupae irrespective of solvent used (Table 2). Mortalities from exposure to Neem leaf extract was less than 80%. Exposure of pupae to the different treatments differed significantly (p <0.001) irrespective of part, geographical source or solvent used in the extraction of P. dodecandra except for the negative control (p >0.05) (Table 3).
|
Table 1 Mean percent mortalities due to exposure of An. gambiae pupae to P. dodecandra plant sourced from the highlands (Eldoret). Mean percent mortalities include standard deviation (Mean ±SD) Note: Mean percent mortality followed by superscript of different letter indicate no significant influence of treatment dose on mortality of exposed pupae; The influence was considered significant at p < 0.05 |
|
Table 2 Mean percent mortalities due to exposure of An. gambiae pupae to P. dodecandra plant sourced from the lowlands (Nyando). Mean percent mortalities include standard deviation (Mean ±SD) Note: Mean percent mortality followed by superscript of different letter indicate no significant influence of treatment dose on mortality of exposed pupae; The influence was considered significant at p < 0.05 |
|
Table 3 F statistics on significant difference in % An. gambiae pupae mortalities due to exposure to extracts of P. dodecandra plus control Note: df depicts degree of freedom; F depicts F statistics in ANOVA; P depicts statistical differences in effectiveness of the treatments. P was considered significant at p < 0.05 |
Water extracts of P. dodecandra was needed in low doses for observed toxicity against pupae compared to ethanol extracts. Toxicity strength of P. dodecandra plant parts extracts was of the order; fruits > leaves of the shoot > leaves of the mid-section irrespective of solvent of extraction or source of P. dodecandra. Dose of treatments did not significantly influence pupae mortality (p > 0.05) except for water extracts of mature green fruits from Nyando (Table 4). All calculated goodness of fit determined by χ2 were less than the χ2 critical value of 22.4 (df = 13; p < 0.05) and therefore the hypothesis of no relationship (H0) between dose and mortality of An. gambiae pupae was retained.
|
Table 4 Estimated lethal concentration that kills 50% (LC50) of ethanol and water extracts of P. dodecandra used against An. gambiae pupae. The estimated LC50 are reported together with standard errors (SE) Note: 1. df = degrees of freedom (n-2); 2. χ2 = chi-square test statistics of relationship between the considered factors; 3. p = level of significance. This was considered significant at p < 0.05; 4. SE = standard error; 5. Columns’ having estimated LC50 superscripted with different letters indicate a significant influence of dose on pupae mortality |
2 Discussions
In the current study it was demonstrated that water extracts of mature green fruits of P. dodecandra was more potent as pupicide compared to ethanol extracts as was extracts of P. dodecandra from the lowlands (Nyando) compared to that from the highlands (Eldoret) irrespective of dose. Plenty of studies exist that demonstrate bioefficacy of plant extracts against vector mosquitoes. For example Carvalho et al. (2003) reported larvicidal activity of the essential oil from Lippiasidoides, against Ae. aegypti Linn, Mann and Kaufman (2012) of essential oil and Jenson et al. (2006) of crude extracts of various plants extracts against different mosquito species in the field of vector control. In all these cases the levels of potency of plant extracts were correlated with type and part of plant and solvent used in the extraction (Jeyabalan et al., 2003). In this study we also demonstrate that crude extracts of P. dodecandra is toxic against An. gambiae pupae irrespective of solvent used in extraction or geographical origin of P. dodecandra plant.
The findings of the current study is also consistent with those of extracts of berries of Endod against Immature filarial vector, Cx. Quinquefasciatus Say (Diptera: Culicidae) (Misganaw et al., 2012), extracts of essential oils from leaves of Plectranthus glandulosus and Callistemon rigidus against pupae of Ae. aegypti and Cx. quinquefasciatus (Pierre et al., 2014) and essential oils from seven plants against pupae of Cx. quinquefasciatus and An. stephensi (Ramar et al., 2014).
The observation that water extracts of mature green fruits of P. dodecandra was more potent than ethanol extracts of the same parts was however inconsistent with an earlier finding of Anupam et al. (2012) and Mgbemena (2010) that concluded that ethanol extracts of bioactive from plants are generally more potent compared to water extracts from the same plants. However, results of this study resonates with an observation made by Anupam et al. (2012), Mgbemena (2010) and Were (2008) on the influence of geographical disposition of a plant on the concentration and distribution of phytochemical therein. The observation that extracts of P. dodecandra sourced from the lowlands (Nyando) was more potent compared to that sourced from the highlands (Eldoret) was however inconsistent with that of Were (2008) in that whereas she demonstrated that extracts of parts of P. dodecandra sourced from the highlands were more potent to that sourced from the lowlands the results herein demonstrates otherwise. The findings herein leads to the conclusion that crude ethanol and water extracts of P. dodecandra is toxic against An. gambiae pupae and is employable as a tool for effective disruption of An. gambiae life cycle.
3 Materials and Methods
3.1 Study area and experimental mosquitoes
The experiments were conducted in the Entomology laboratory at Centre for Global Health Research/Kenya Medical Research Institute (CGHR/KEMRI). An. gambiae mosquitoes maintained at the laboratories were used. Mosquito culture was done following standard techniques (Das et al., 2007) at temperatures of 28-30°C, relative humidity of 70-80% and photoperiod of 12:12 (L:D).
3.2 Plant extracts preparation
Phytolacca dodecandra plant parts were sourced from Eldoret [+0.518829ON, 35.284927OE] and Nyando [-0.250393ON, 34.870190OE] and Azadirachta indica (Neem) from Nyando [-0.250393ON, 34.870190OE]. The plant parts were identified, voucher specimen number JOY2012/001 for P. dodecandra and JOY2012/002 for A. indica issued and thereafter deposited in the herbarium at the School of Biological Sciences, University of Nairobi, Kenya. The plant parts were prepared and extracted using standard procedure describe by Das et al. (2007) and Parekh et al. (2005). The extracts were then kept in airtight glass bottles to serve as stock quantity.
3.3 Pupicidal bioassays
Twenty early stage pupae (pupae that metamorphosed from L4 larvae within a two hour window) of An. gambiae were placed individually using a dropper in transparent plastic containers measuring 6 cm top × 5.7 cm bottom × 3.5 cm height. The containers with pupa were arranged in sets of threes and each set of containers received approximately 33 mls of a particular concentration of treated rain water.
The concentrations were prepared by taking 80 mgs of freeze-dried stock, dissolving it in 200mls of rain water and serially diluting to obtain concentrations of 40, 20, 10, 5 and 2.5 mg in 100 mls of rain water. Positive (ethanol and water extracts of Neem) and negative controls (untreated rain water) were also set simultaneously. The set up was replicated ten times.
The mouth of each container was covered with mosquito netting to prevent emerged adult mosquito from escape. The exposed pupae were left to stay in the treatments on experimental tables overnight and mortality rate registered after 24 hours post exposure. Pupae mortality was calculated for each concentration using the formula;
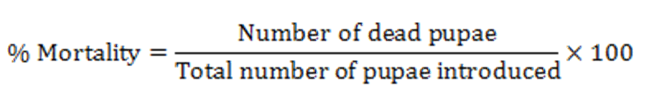
Mortality was corrected using Abbotts formula (Abbott, 1925).
.png)
In addition, standard WHO procedures were used to assess effectiveness of the extracts as pupicide at a threshold mortality rate of > 80% (WHO, 2005).
3.4 Data analysis
Data obtained from the bioassays was entered in excel spreadsheets and the relationship between pupicidal effect of the extracts with part of P. dodecandra plant used and concentration determined using descriptive statistics. One way analysis of variance (ANOVA) was used to determine the level of significance of the effects of treatments on pupae mortality. Regression (probit) analysis was used to calculate the lethal concentration (LC50) and χ2 statistics of the extracts used. Results on concentrations of extracts were expressed as mean ± SD and those on effect of dose expressed as mean ± SE of three replicates in each treatment. P-values were considered significant at p < 0.05. All statistical analysis was performed using Statistical Package for Social Scientist (SPSS) version 16.
Authors’ contributions
Y.J.O., conceived, designed the experiments, wrote manuscript and analyzed the data, K.J.J., and M.C., conducted the experiments. . All authors read and corrected the manuscript.
Competing interest
The authors declare that they have no competing interest.
Acknowledgments
I thank Mr. Richard Amito, Mr. Dalton Ochieng’, Ms Charlotte Awuor, Ms. Patience Akoth and Mr. Trevor Omondi for culturing mosquitoes used in this study. I also thank Centre for global Health Research /Kenya Medical Research Institute (CGHR/KEMRI) for laboratory space, mosquitoes and equipments for conducting the experiments and National Commission for Science Technology and Innovation (NACOSTI) for funding the project (Grant contract # NCST/5/003/3rd Call PhD/056 to YJO).
Abbott W., 1925, A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18: 265-267
https://doi.org/10.1093/jee/18.2.265a
Ansari M., Mittal P., Razdan R., and Sreehari U., 2005, Larvicidal and mosquito repellent activities of pine (Pinuslongifolia, Family: Pinaceae) oil, Journal of Vector Borne Diseases, 42: 95-99
PMid: 16294807
Anupam G., Nandita C., and Goutam C., 2012, Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research, 135: 581-598
Balu S., Gokulakrishnan J., Elanchezhiyan K., and Deepa J., 2015, Mosquito larvicidal, ovicidal and pupicidal activities of annona reticulatalinn (annonaceae) against aedes aegypti (Linn.), anopheles stephensi Liston and culex quinquefasciatus (Say) (diptera : culicidae), International Journal of Recent Scientific Research,6(2): 2690-2696
Brattsten L., and Hamilton G., 2012, Insecticides recommended for mosquito control in New Jersey in 2012, New Jersey Agricultural Experiment Station: Publication No. P-08001-01-12, pp.18
Carvalho A., Melo V., Craveiro A., Machado M., Bantim B., and Rabelo E., 2003, In: Memoir Institute de Oswaldo Cruz, 98: 569-571
https://doi.org/10.1590/S0074-02762003000400027
PMid: 12937776
Cavalcanti E., De Morais C., Lima M., and Santana E., 2004, Larvicidal activity of essential oils from Brazilian plants against Aedesaegypti L. Memoir Institute de Oswaldo Cruz, 99: 541–544
https://doi.org/10.1590/S0074-02762004000500015
PMid: 15543421
Choochote W., Chaiyasit D., Kanjanapothi D., Rattanachanpichai E., Jitpakdi A., Tuetun B., and Pitasawat B., 2005, Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae). Journal of Vector Ecology, 30: 302–309
PMid: 16599168
Das N.G., Goswami D., and Rabha B., 2007, Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. Journal of Vector Borne Diseases, 44:145-148
PMid: 17722869
Elumalai K., Dhanasekaran S., Anandan A., Krishnappa K., Gokulakrishnan J., and Elangovan A., 2012b, Mosquitocidal activities of Abruspreca torius L (Fabaceae) against chickungunya vector, Aedes aegypti (L.) and Japanese encephalitis vector, Culex tritaeniorhynchus (Giles) (Diptera: Culicidae). International Journal of Current Research in Agriculture, 2(7), 28 – 33
Elumalai K., Dhanasekaran S., Krishnappa K., Gokulakrishnan J., and Elangovan A., 2012a, Larvicidal, ovicidal and pupicidal activity of Eranthemum roseum (Vahl) R. Br. Against malarial vector mosquito, Anopheles stephensi (Liston) (Diptera : Culicidae) International Journal of Current Life Science, 2(7): 31 – 38
Elumalai K., Mathivanan T., Dhanasekaran S., Packiam M.S., and Krishnappa K., 2013, Mosquitocidal Activities of Isolated Compounds from the Essential Oil of Pulchea indica L. (Asteraceae) against five Medically Important Human Vector Mosquitoes (Diptera: Culicidae). Instanbul Journal of Chemistry, 3(1)
Gokulakrishnan J., Balu S., Elumalai K., and Krishnappa K., 2012, Mosquito larvicidal and ovicidal efficacy of Ariitolochia indica Linn (Aristolochiaceae) leaf extracts against malarial vector mosquito Anopheles stephensi Liston (Diptera: Culicidae). International Journal of Current Life Science, 2 (10): 48-52
Jenson T.G., Palsson K., and Borg-Karlson A.K., 2006, Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. Journal of Medical Entomology, 43: 113-119
https://doi.org/10.1093/jmedent/43.1.113
Jeyabalan D., Arul N., and Thangamathi P., 2003, Studies on effects of Pelargonium citrosa leaf extracts on malarial vector, Anopheles stephensi, Liston. Bioresource Technology, 89:185-189
https://doi.org/10.1016/S0960-8524(03)00036-1
Krishnappa K., and Elumalai K., 2013, Mosquitocidal properties of Basella rubra and Cleome viscose against Aedes aegypti (Linn.) (Diptera :Culicidae). European Review of Medical and Pharmaceutical Science, 17: 1273-1277
PMid: 23690200
Mann R.S., and Kaufman P.E., 2012, Natural product pesticides: their development, delivery and use against insect vectors. Mini-review of Organic Chemistry, 9: 185-202
https://doi.org/10.2174/157019312800604733
Mgbemena I.C., 2010, Comparative evaluation of larvicidal potentials of three plant extracts on Aedes aegypti. Journal of American Science, 6: 435-440
Misganaw N., Moges S., Tadele M., Tesera M., Temesgen T., and Raja N., 2012, Evaluation of Multi Potential Bioactive Endod, Phytolacca dodecandra (L’ Herit) Berries Extracts Against Immature Filarial Vector Culex quinquefasciatus Say (Diptera: Culicidae). Research Journal of Environmental Earth Science, 4(7): 697-703
Ndione D.R., Ndiaye M., Faye O., Afoutou J.M., and Dieye A., 2013, Larvicidal and cytopathologic effects of Suneem 1% (neem: Azadirachta indica, A. (Juss, Meliaceae) on mosquitoes vectors of diseases. Top class Journal of Herbal Medicine, 2(3): 43-58
Omolo M., Okinyo D., Ndiege I., Lwande W., and Hassanali A., 2004, Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry, 2797–2802
https://doi.org/10.1016/j.phytochem.2004.08.035
PMid: 15474566
Parekh J., Jadeja D., and Chanda S., 2005, Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turkish Journal of Biology, 29: 203-210
Pierre D.Y.S., Okechukwu E.C., Lame Y., and Nchiwan N.E., 2014, Larvicidal and Pupicidal Activities of Plectranthus glandulosus and Callistemon rigidus Leaf Essential Oils against Three Mosquito Species. Journal of Mosquito Research, 4(2): 5-14
Pitasawat B., Choochote W., Tuetun B., Tippawangkosol P., Kanjanapothi D., Jitpakdi A., and Riyong, D., 2003, Repellency of aromatic turmeric curcuma aromatica under laboratory and field conditions. Journal of Vector Ecology, 28: 234–240
PMid: 14714673
Praveen K., Renuka J., Shweta J., and Archana S., 2012, A review on biological and phytochemical investigation of plant genus callistimon. Asian Pacific Journal of Tropical Biomedicine, S1906-S1909
Pravin Y., Saranya M., Sivakumar T., Mahendran S., Mohanraj R.S., and Dhanakkodi B., 2015, Larvicidal, pupicidal, ovicidal activity and GC-MS analysis of Spathodeacam panulata P. Beauv. (Bignoniaceae) acetone leaf extract against the dengue vector mosquito Aedes aegypti (Diptera: Culicidae). International Journal of Current Research and Academic Reviews, 3(5): 92-111
Ramar M., Ignacimuthu S., and Paulraj M.G., 2014, Bio-efficacy of pupicidal activity of some plant essential oils on Culex quinquefasciatus and An. stephensi. The International Journal of Biotechnology, 3(8): 104-114
Ramar M., Paulraj M.G., and Ignacimuthu S., 2013, Preliminary screening of plant essential oils against larvae of culex quinquefasciatus Say (Diptera: Culicidae). African Journal of Biotechnology, 12(46): 6480-6483
https://doi.org/10.5897/AJB2013.12967
Traboulsi A., El-Haj S., Tueni M., Taoubi K., Nader N., and Mrad A., 2005, Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens Molestus (Diptera: Culicidae). Pest Management Science, 61: 597–604
https://doi.org/10.1002/ps.1017
PMid: 15662650
Were P.K., 2008, Efficacy of Phytolacca dodecandra on Anopheles gambiae mosquito larvae. [PhD dissertation] School of Environmental Studies, Moi University, Eldoret, pp. 78-143
WHO, 2005, Guidelines for laboratory and field testing of mosquito larvicides, WHO/CDS/WHOPES/ GCDPP/2005: 13
Yang Y., Lee S., Lee H., Kim M., Lee S., and Lee H., 2002, Apiperidine amide extracted from piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. Journal of Agriculture and Food Chemistry, 50: 3765–3767
https://doi.org/10.1021/jf011708f
PMid:12059157
Yugi J.O., Okeyo-Owuor J.B., Auma C.A., Juma J.I., and Vulule J.M., 2015, Larviciding potency of water and ethanol extracts of Phytolacca dodecandra (L’ Herit) on Anopheles gambiae (Diptera: Culicidae). Journal of Mosquito Research, 5(2): 1- 6
https://doi.org/10.5376/jmr.2015.05.0002
Yugi J.O., Okeyo-Owuor J.B., and Omondi D.O., 2016, Adulticidal effect of crude ethanol extract of Phytolacca dodecandra on Anopheles gambiae. Journal of Mosquito Research, 6(1): 1-5
. PDF(419KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jared Owiti Yugi
. Joyce J. Kiplimo
. Christopher O. Misire
Related articles
. Anopheles gambiae
. Ethanol
. Phytolacca dodecandra
. Pupicide
. Water
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)