Research Article
Larvicidal Efficacy of Cleistanthus patulus Muell. Arg. (Euphorbiaceae) Leaf Extract against Filarial Vector Culex quinquefasciatus (Say 1823) 
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2017, Vol. 7, No. 12 doi: 10.5376/jmr.2017.07.0012
Received: 25 May, 2017 Accepted: 17 Jul., 2017 Published: 28 Jul., 2017
Pal J.K., Singh A., and Chandra G., 2017, Larvicidal efficacy of Cleistanthus patulus Muell. Arg. (Euphorbiaceae) leaf extract against filarial vector Culex quinquefasciatus (Say 1823), 7(12): 96-103 (doi: 10.5376/jmr.2017.07.0012)
As resistance against synthetic insecticides has been increasing day by day, mosquito control becomes a great problem around the world. So it is crucial to manage the vector population to overcome the mosquito borne diseases. The present study was executed to assess the larvicidal activities of Cleistanthus patulus leaf extract against filarial vector Culex quinquefasciatus. Method: Crude extracts of C. patulus mature leaf with different concentration gradients ranging from 0.1% - 0.5% were applied against all the larval instars of Cx. quinquefasciatus. Three solvent extractives namely petroleum ether, ethyl acetate and acetone were applied in different graded concentrations against all the larval instars. Through log-probit analysis LC50 and LC90 values were determined. Regression and ANOVA analyses were done for further statistical justification. Activities of bioactive fraction against the non-target organism were carried out in laboratory condition. In addition to these, a qualitative phytochemical analysis of leaf extract was also done. Result: The highest mortality was observed at 0.5 concentration of crude extract against all the larval instars after 72 hours of exposure. Among three used solvent extracts ethyl acetate exhibited the best larvicidal potentiality against target mosquito. 1st instar larvae showed 100% mortality at 250 ppm concentration after 48 hours of exposure. Preliminary qualitative phytochemicals analyses of leaf extract revealed the presence of tannin, steroid and flavonoid as secondary metabolites. Non-target organism was found non-responsive to both extracts. Conclusion: Above experiment indicates that the leaf of C. patulus has the prospective to be used as larvicidal agent against Cx. quinquefasciatus.
Background
Mosquito, the wing devil transmits various deadly diseases like malaria, filariasis, dengue, different types of encephalitis etc. Each year almost 700 million people get infected by these mosquito borne diseases (Taubes, 1997). In India every year about 40 million people are affected by mosquito borne diseases (Ghos, 2012).
Different chemically synthesised insecticides were used to control mosquito population for a long time. But, as the resistance power of mosquito against these chemically synthesised insecticides has increased, the control of mosquito becomes more complicated. Besides, synthetic insecticides are not cost effective, biodegradable, target specific and eco-friendly in nature. They harmfully affect the environment by contaminating soil, water & air (Singha et al., 2012). So, nowadays researchers are trying to find out an alternative of these synthetic insecticides. Plants are the source of uncountable no of phytochemicals. Plant secondary metabolites, such as terpene, tannin, flavonoid, lignin, saponin, cardiac glycoside, alkaloid etc. which serve in plant defense, are mainly responsible for insecticidal properties (Hassan Adeyemi, 2010). Many researchers have proved that plant derived phytochemicals can be used as an alternative of chemically synthetic insecticides for their cost effective, biodegradable, target specific and eco-friendly nature (Bhattacharya et al., 2014a; Singh et al., 2015, Pal et al., 2016).
Cx. quinquefasciatus acts as a major vector of lymphatic filariasis (Hati et al., 1989). About 120 million people all over the world have been infected by this tropical disease and it becomes chronic among 44 million people (Otten et al., 1997; Bernhard et al., 2003). As per WHO (1992) about 90 million people around the globe are infected with Wuchereria bancrofti that dwells in lymphatic system and ten times more people are at the risk of being infected. Particularly in India, 25 million people suffer from microfilariasis and 90 million people from filarial disease manifestation (Kovendan et al., 2012).
Cleistanthus patulus (Roxb.) Müll. Arg. is a common deciduous plant in India and Sri Lanka. It belongs to the family Euphorbiaceae (Singh et al., 2014).This plant is 2-5 m tall, leaves alternate, flowers unisexual, sepals and petals each 5 in number. Cleistanthin A and Cleistanthin B phytoconstituents of Cleistanthus have the diuretic and anticancerous properties (Parasuraman and Raveendran, 2012).
The objective of the present study was to find out the larvicidal efficacy of crude and solvent extracts of mature leaves of C. patulus against Cx. quinquefasciatus as a target species.
1 Materials and Methods
1.1 Collection of plant materials
Mature unspotted leaves of C. patulus were collected during June-July 2016 from its growing region at the surrounding areas of Gonpur (24.0654° N, 87.6741° E), Birbhum, India. After identification of the plant, a voucher specimen (Voucher No. GCZJKP-08) was submitted as herbarium at the Mosquito, Microbiology and Nanotechnology Research Units, Department of Zoology, The University of Burdwan.
1.2 Collection of larvae and colony set up
The larvae of Cx. quinquefasciatus were collected from the drains around the Burdwan University campus (23°16´N, 87°54´E). To set up a larval colony larvae were kept in a plastic tray filled with dechlorinated tap water with appropriate hygiene. As complementary food a mixture of dog biscuits, algae and Brewer’s yeast powder (1:1:3) were given to these larvae (Kamaraj et al., 2009). The colony was maintained at the temperature of 27 (± 2)°C and the relative humidity 80 % - 85 % under photoperiod of light and dark cycles in the ratio of 14:10 per day. Within the tray, larvae became pupae, and after that those were transferred to an insectary (45×45×40 cm) for adult immergence. As supplement food 10% glucose solution and multivitamin syrup soaked cotton ball were given to the adult mosquitoes. On the 5th day of rearing an anesthetized pigeon was supplied for the blood meal of adult females. For laying eggs of adult females a Petri dish filled with water and crumpled filter papers were put in the rearing insectary. Eggs were allowed to hatch in laboratory condition and it was maintained in a separate container. Thus F1 generation was obtained and a healthy mosquito colony was formed. Adult mosquitoes were identified with the help of the key provided by Christophers (1933), Barraud (1934) and Chandra (2000).
1.3 Preparation of crude extract
For the preparation of crude extract mature and unspotted leaves of C. patulus were collected and then these were cleaned well with tap water and then by distilled water. After soaking the leaves with a paper towel these fresh leaves were crushed in a grinder and then filtered with Whatman’s No-1 filter paper. The crude extract was preserved as stock solution for further test. By adding distilled water the required concentrations were prepared.
1.4 Differential solvent extraction
Fresh and unspotted leaves were dried up in room temperature. The dried leaves were chopped in small pieces and these pieces of leaves were put into thimble of Soxhlet apparatus. Two thousand (2000) ml of each solvent, one after another, was added to the still pot of Soxhlet apparatus. The time for extraction was fixed up for 72 hours with 8 hours maximum per day. Three solvents (petroleum ether, ethyl acetate and acetone) were passed in non-polar to polar fashion. After evaporation with rotary evaporator extracts were kept at 4°C in a refrigerator for further use.
1.5 Dose-response larvicidal bioassay
In accordance with the standard protocol of WHO (2005) larvicidal bioassays were performed in the laboratory. A number of 25 larvae of different instars were transferred to Petri dishes that contain 100 ml of tap water. Crude extract of graded concentration 1000 ppm, 2000 ppm, 3000 ppm, 4000 ppm and 5000 ppm were applied to different Petri dishes. Likewise graded concentration (50 ppm, 100 ppm, 150 ppm, 200 ppm and 250 ppm) of three solvent extractives were also prepared and applied to different Petri dishes. All the experiments were done thrice on different days at room temperature. Larval mortality was observed after 24 h, 48 h, 72 hours of exposure respectively. Dead larvae were counted as their failure of movement when they were pinned with a needle to their siphon of the cervical region.
1.6 Phytochemical analysis of the plant extracts
Phytochemical analysis of crude extract of the leaves were performed according to the standard method of Sofowara (1993), Trease and Evans (1989) and Harborne (1973). Crude extract was examined for whether the secondary metabolites like tannins, saponin, steroid, flavonoid and terpenoid are present or absent.
1.7 Larvicidal bioassay on a non target organism:
Diplonychys annulatum was used as a non-target organism as they are present in the similar habitat with mosquito larvae. D. annulatum nymphs were tested to a concentration of crude extract and ethyl acetate extractive that was similar to that of median lethal concentration (LC50) of 3rd instar of mosquito larvae at 24 hours. Mortalities and other abnormalities such laziness of swimming activity was observed after 24h, 48h and 72h of exposure.
1.8 Statistical analysis
Abbott’s formula (Abbott, 1925) was applied to rectify the percent mortality throughout the observation. Regression analysis was done by using “MS EXCEL 2007”. Probit and three way random AVONA analyses were done by using “STAT PLUS 2009 (Trial version)”.
2 Results
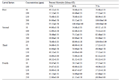
C. patulus was found to be efficient mosquito larvicidal agent against Cx. quinquefasciatus larvae. 100% mortality was found against 1st instars larvae at 4000 ppm concentration of crude extract after 72 h of exposure (Table 1). Among three different solvent extractives, ethyl acetate extractive exhibited highest mosquito larvicidal potentiality against Cx. quinquefasciatus. Cent percent mortality was found in 1st instar larvae at 200 ppm concentration of ethyl acetate extractive at 72 h of post exposure (Table 2). The mortality gradually increased with an increase of exposure time in each larval instar. The result of log probit analysis (95% confidence level) reveals the reversely proportional relation of time of exposure and values of LC50 and LC90. Lowest LC50 and LC90 value were found at 72 h of exposure in each larval instar. Lowest LC50 and LC90 values were found against 1st instars larvae after 72h of exposure followed by 2nd, 3rd and 4th instars larvae. In crude extract lowest LC50 and LC90 values were 800 ppm & 2400 ppm (95% confidence level) respectively (Table 3). In ethyl acetate extractive lowest LC50 and LC90 values were 33.28 ppm & 122.37 ppm (95% confidence level) respectively (Table 4). The results of regression analysis revealed that the mortality rate (Y) was positively correlated with the concentration of exposure (X) having a regression coefficient (R2) close to 1 in each case (Table 3; Table 4).The result of the three-way factorial ANOVA (Table 5) of ethyl acetate extractive of leaves of C. patulus carried out with different concentrations, different time intervals and different instars revealed significant difference in larval mortality (p<0.05) with respect to these three parameters. No mortality or abnormality related to sluggishness or swimming activity was observed in non-target organism, D. annulatum after 72 h of exposure. Primary qualitative phytochemical analysis reveals that the leaves contain tannin, steroid and flavonoid as secondary metabolites.
|
Table 1 Percent mortality of Cx. quinquefasciatus larvae using crude extract of C. patulus leaves |
|
Table 2 Percent mortality of Cx. quinquefasciatus larvae using ethyl acetate extracts of C. patulus leaves |
|
Table 3 Probit analysis and regression analysis of mortality rates of larvae of Cx. quinquefasciatus in crude extract of leaf of C. patulus |
|
Table 4 Probit analyses and regression analyses of mortality rates of larvae of Cx. quinquefasciatus in ethyl acetate extract of leaf of C. patulus |
|
Table 5 Three way ANOVA of mortality rates of different larval instars, hours of exposure and concentration as variables |
3 Discussion
Nowadays conventional synthetic insecticides are obsolete because mosquitoes become resistance among those insecticides. So, vector control is now facing a great problem. Therefore, some novel measures should be taken to counter against this menace. Insecticide from botanical sources may be suitable substitute for synthetic insecticides because they are more safe, degradable and easily available (Hossain et al., 2009; Mallick et al., 2015; 2016). Different plants from different families have been reported in many reputed journals for mosquito larvicidal (Haldar et al., 2011; Singha and Chandra, 2011; Singh Ray et al., 2014; Bhattacharya and Chandra 2013, 2015; Mondal et al., 2016), pupicidal, repellent, adulticidal and smoke toxic (Singha et al., 2011b; Bhattacharya and Chandra 2014; Rawani et al., 2012) properties. Larval control is easier than control of adults due to their low mobility and confinement to water bodies.
In this experiment, the result reveals that leaf extract of C. patulus has effective larvicidal activity against Cx. quinquefasciatus larvae. Among the used solvents ethyl acetate shows the best larvicidal property. Tested non target organism is completely safe from the effect of crude and solvent extractives. Qualitative phytochemical analyses reveal that the leaves contain tannin, steroid and flavonoid as secondary metabolites which may be responsible for the larvicidal property.
Many researchers had documented different herbal solvent extractives as mosquito larvicidal agent in various reputed journals. Bagavan et al. (2008) reported that ethyl acetate extractive of Acyranthes aspera leaf showed cent percent larval mortality against Cx. quinquefasciatus at 1000 ppm concentration. In comparison, we found 100% larval mortality in only 250 ppm concentration after 72 h of exposure. Also, Kundu et al. (2013) reported that 480ppm concentration of ethyl acetate solvent extract of seed coat of Cassia sophera was responsible for cent percent larval death of Cx. quinquefasciatus. In our experiment the dose for cent percent larval death of Cx. quinquefasciatus was very low. Singha Ray et al. (2015) published that ethyl acetate extractive of Capparis zeylanica leaf exhibited larvicidal efficiency against Cx. quinquefasciatus with lowest LC50 and LC90 values were 12.44 ppm & 33.88 ppm respectively. In our experiment in ethyl acetate extractive lowest LC50 and LC90 values were 33.28 ppm & 122.37 ppm respectively. Chloroform: methanol (1:1 v/v) extractives of Ravenala madagascariensis leaves (Bhattacharya et al., 2014b) and Solanum nigrum berry (Rawanietal, 2013) showed potent larvicidal property against Cx. quinquefasciatus. Acetone extractives of leaf of Nicotiana plumbaginifolia (Singh et al., 2016), hexane extract of flower of Nerium oleander (Raveen et al., 2014), petroleum ether and N-butanol extracts of Cassia occidentalis (Kumar et al., 2014), methanol extract of Cedrus deodara stem bark (Rahuman et al., 2009) also showed potent larvicidal effect against same mosquito species.From different plant parts, several secondary metabolites such as alkaloids, phenolics, terpenoids, essential oils etc. have been reported earlier for their mosquitocidal activities (Shaalan et al., 2005).
In conclusion, this is the first ever report about the larvicidal efficacy of leaf extracts of C. patulus against Cx. quinquefasciatus. However, further study is needed to know the chemical constitution of the active fraction involved and its actual mode of action in the target species.
Abbott W.S., 1925, A method of computing the effectiveness of an insecticide, Journal of Economic Entomology, 18: 265-266
https://doi.org/10.1093/jee/18.2.265a
Bagavan A., Rahuman A.A., Kamaraj C., and Kannappan G., 2008, Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae), Parasitology Research, 103: 223-229
https://doi.org/10.1007/s00436-008-0962-z
PMid: 18392726
Barraud, P.J., 1934, The Fauna of British India, including Ceylon and Burma, Diptera Vol -IV. Taylor and Francis London, 1-455
Bernhard L., Bernhard P., and Magnussen P., 2003, Management of patients with lymphoedema caused by filariasis in north-eastern Tanzania: alternative approaches, Physiotherapy, 89: 743-749
https://doi.org/10.1016/S0031-9406(05)60500-7
Bhattacharya K., Chandra I., Kundu P., Ray S., Halder D., and Chandra G., 2014a, Larval control of Culex vishnui group through bio-active fraction of traveller’s tree, Ravenala madagascariensis Sonn. (Strelitziaceae), Journal of Mosquito Research, 4(15): 1-6
https://doi.org/10.5376/jmr.2014.04.0015
Bhattacharya K., Burman S., Nandi S., Roy P., Chatterjee D., and Chandra G., 2014b, Phytochemical extractions from the leaves of Ravenala madagascariensis from Sundarban area and its effect on southern house mosquito (Culex quinquefasciatus Say 1823) larvae, Journal of Mosquito Research, 4(12): 1-6
Bhattacharya K., and Chandra G., 2013, Bioactivity of Acyranthes aspera (Amaranthaceae) Foliage against the Japanese Encephalitis Vector Culex vishnui Group, Journal of Mosquito Research, 3(13): 89-96
https://doi.org/10.5376/jmr.2013.03.0013
Bhattacharya K., and Chandra G., 2014, Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrata L. (Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae), Asian Pacific Journal of Tropical Disease, 4 (Suppl 1): S226-S232
https://doi.org/10.1016/S2222-1808(14)60444-8
Bhattacharya K., and Chandra G., 2015, Biocontrol efficacy of Operculina turpethum (L.) (Convolvulaceae) leaf extractives against larval form of malarial mosquito Anopheles stephensi (Liston 1901), International Journal of Pharma and Bio Sciences., 6(3): (B) 460-468
Chandra G., 2000, Mosquito, Sribhumi Publication Co. p 1-102
Christophers, S.R., 1933, The Fauna of British India, including Ceylon and Burma, Diptera Vol -V. Taylor and Francis London: 360
Ghosh A., Chowdhury N., and Chandra G., 2012, Plant extract as potential mosquito larvicide, Indian Journal of Medical Research, 135(5): 581-598
Haldar K.M., Ghosh P., and Chandra G., 2011, Evaluation of target specific larvicidal activity of the leaf extract of Typhonium trilobatum against Culex quinquefasciatus Say, Asian Pacific Journal of Tropical Biomedicine, 1(2): S199-203
https://doi.org/10.1016/S2221-1691(11)60156-1
Harborne J.B., 1973, Phytochemical methods, Chapman and Hall, Ltd, London: 49-188
Hassan Adeyemi M.M., 2010, The potential of secondary metabolites in plant material as deterrents against insect pests: A review, African Journal of Pure and Applied Chemistry, 4(11): 243-246
Hati A.K., Chandra G., Bhattacharyya A., Biswas D., Chatterjee K.K., Dwibedi H.N., 1989, Annual transmission potential of bancroftian filariasis in an urban and a rural area of West Bengal, India, The American journal of tropical medicine and hygiene, 40(4): 365-367
https://doi.org/10.4269/ajtmh.1989.40.365
PMid: 2653062
Hossain E., Rawani A., Chandra G., Mandal S.C., and Gupta J.K., 2011, Larvicidal activity of Dregea volvubilis and Bombax malabaricum leaf extracts against the filarial vector Culex quinquefasciatus, Asian Pacific Journal of Tropical Medicine, 436-441
https://doi.org/10.1016/S1995-7645(11)60121-1
Kamaraj C., Bagavan A., Rahuman A.A., and Zahir A.A., Elango G., and Pandiyan G., 2009, Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae), Parasitology Research, 104:1163-71
https://doi.org/10.1007/s00436-008-1306-8
PMid: 19085005
Kovendan K., Arivoli S., Maheshwaran R., Baskar K., 2012, Larvicidal efficacy of Sphaeranthus indicus, Cleistanthus collinus and Murraya koennigii leaf extracts against filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae), Journal of Parasitology Research, doi10.1007/s00436-012-2927-5
https://doi.org/10.1007/s00436-012-2927-5
Kumar D., Chawla R., Dhamodaram P., and Balakrisnan N., 2014, Larvicidal Activity of Cassia occidental is (Linn.) against the Larvae of Bancroftian Filariasis Vector Mosquito Culex quinquefasciatus, Journal of Parasitology Research, 5
Kundu M., Rawani A., and Chandra G., 2013, Evaluation of Mosquito Larvicidal Activities of Seed Coat Extract of Cassia sophera L., Journal of Mosquito Research, 3(11): 76-81
https://doi.org/10.5376/jmr.2013.03.0011
Mallick S., Mukherjee D., and Chandra G., 2015, Evaluation of Larvicidal Efficacy of Acetone Leaf Extracts of Annona reticulata Linn. Against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae), Journal of Mosquito Research, 5(9): 1-7
https://doi.org/10.5376/jmr.2015.05.0009
Mallick S., Mukherjee M., Singha ray A., and Chandra G., 2016, Larvicidal Efficacy of Fruit peel Extracts of Citrus maxima Linn. Against Culex quinquefasciatus, Journal of Mosquito Research, 6(20): 1-8
Mondal R.P., Singh A., Ghosh A., and Chandra G., 2016, Studies on larvicidal activity of some plant extracts against filarial vector Culex quinquefasciatus, Journal of Mosquito Research, 6(7): 1-6
Otten E.A., Duke B.O., Karam M., and Behani K., 1997, Strategies and tools for the control/elimination of lymphatic filariasis, Bulletin of the World Health Organization, 75(6): 491-503
Pal J.K., Singh A., Rawani A., and Chandra G., 2016, Larvicidal activity of Tinosporacrispa (Menispermaceae) fruit extract against filarial vector Culex quinquefasciatus, Journal of Mosquito Research, 6(35): 1-8
https://doi.org/10.5376/jmr.2016.06.0035
Parasuraman S., and Raveendran R., 2012, Diuretic effects of Cleistanthin A and Cleistanthin B from the leaves of Cleistanthus collinusin Wistar rats, Journal of Young Pharmacists, 4:73-7
https://doi.org/10.4103/0975-1483.96616
PMid: 22754257 PMCid:PMC3385220
Rahuman A.A., Bagavan A., Kamaraj C., Saravanan E., Zahir A.A., and Elango G., 2009, Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae), Parasitology Research, 104
https://doi.org/10.1007/s00436-009-1337-9
Raveen R., Kamakshi K.T., Deepa M., Arivoli S., and Tennyson S., 2014, Larvicidal activity of Nerium oleander L. (Apocynaceae) flower extracts against Culex quinquefasciatusSay (Diptera: Culicidae), International Journal of Mosquito Research, 1 (1): 38-42
Rawani A., Chowdhury N., Ghosh A., Laskar S., and Chandra G., 2013, Mosquito larvicidal activity of Solanum nigrum berry extracts, Indian Journal of Medical Research, 137: 972-976
Rawani A., Ghosh A., Laskar S., and Chandra G., 2012, Aliphatic Amide from Seeds of Carica papaya as Mosquito Larvicide, Pupicide, Adulticide, Repellent and Smoke Toxicant, Journal of Mosquito Research, 2(2): 8-18
https://doi.org/10.5376/jmr.2012.02.0002
Shaalan E.A.S., Canyonb D., Younesc M.W.F., Abdel-Wahaba H., and Mansoura A.H., 2005, A review of botanical phytochemicals with mosquitocidal potential, Environment International, 3: 1149-66
Singh A., Bhattacharya K., and Chandra G., 2015, Efficacy of Nicotiana plumbaginifolia (Solanaceae) leaf extracts as larvicide against malarial vector Anopheles stephensi Liston 1901, International Journal of Pharma and Bio Sciences, 6(1): (B) 860-868
https://doi.org/10.1016/j.envint.2005.03.003
PMid: 15964629
Singh A., Bhattacharya K., Singh Ray A., and Chandra G., 2016, Larvicidal efficacy of mature leaf extract of Nicotiana plumbaginifolia Viv. (Solanaceae) against southern house mosquito, International Journal of Pharma and Bio Sciences, 7(2): (B) 162-167
Singh B., Borthakur S. K., and Phukan S.J., 2014, Cleistanthus nokrensis (Euphorbiaceae), a New Species from Indian, Himalaya, Taiwania, 59 (3): 197-205 DOI:10.6165/tai.2014.59.197
Singh Ray A., Bhattacharya K., and Chandra G., 2015, Target specific larvicidal effect of Capparis zeylanica L. (Capparaceae) foliages against filarial vector Culex quinquefasciatus, International Journal of Pharma and Bio Sciences, 6(3): (B) 139-148
Singh Ray A., Bhattacharya K., Singh A., and Chandra G., 2014, Larvicidal Activity of Nelumbo nucifera Gaertn. (Nymphaeaceae) against Anopheles stephensi (Liston 1901) and its Effect on Non-target Organisms, Journal of Mosquito Research, 4(10): 1-7
https://doi.org/10.5376/jmr.2014.04.0010
Singha S., Adhikari U., Ghosh A., and Chandra G., 2012, Mosquito larvicidal potentiality of Holoptelea integrifolia leaf extract against Japanese encephalitis vector, Culex vishuni group, Journal of Mosquito Research, 2(4): 25-31
Singha S., and Chandra G., 2011a, Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi, Asian Pacific Journal of Tropical Biomedicine, 288-293
https://doi.org/10.1016/S1995-7645(11)60088-6
Singha S., Banerjee S., and Chandra G., 2011b, Synergistic effect of Croton caudatus (fruits) and Tiliacora acuminate (flowers) extracts against filarial vector Culex quinquefasciatus, Asian Pacific Journal of Tropical Biomedicine, 1(2): S159-S164
https://doi.org/10.1016/S2221-1691(11)60147-0
Sofowora A., 1993, Medicinal plants and Traditional medicine in Africa, Spectrum Books Ltd, Ibadan, Nigeria, 289
Trease G.E., and Evans W.C., 1989, Pharmacognosy, 11th Edn, BrailliarTiridel can, Macmillian Publishers, pp 568
Taubes G., 1997, A mosquito bites back, New York Times Magazine, 24 August, pp 40-46
World Health Organization, 1992, Lymphatic filariasis: the disease and its control, 5th report of the WHO Expert Committee on Filariasis, Technical Report Series, p 821
World Health Organization, 2005, Guidelines for laboratory and field testing of mosquito larvicides, WHO, Geneva WHO/CDS/WHOPES/GCDPP/ 13 pp
. PDF(348KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Goutam Chandra
. Jibon Kumar Pal
. Aniket Singh
Related articles
. Cleistanthus patulus
. Culex quinquefasciatus
. Larvicide
. Non-target organism
Tools
. Email to a friend
. Post a comment
.gif)