Research report
Effect of Habitat Modifications on Predation Potential of Anisops sardea (Hemiptera: Notonectidae) against Larvae of Culex vishnui, Vector of Japanease Encephalitis 
2. Mosquito and Microbiology Research Units, The University of Burdwan, West Bengal, India
3. Department of Mathematics, Bankura Christian College, West Bengal, India
4. Department of Zoology, Bankura Christian College, West Bengal, India
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2016, Vol. 6, No. 30 doi: 10.5376/jmr.2016.06.0030
Received: 12 Sep., 2016 Accepted: 19 Sep., 2016 Published: 25 Nov., 2016
Rajendra P.M., Goutam C., Subhasis B., and Anupam G., 2016, Effect of habitat modifications on predation potential of Anisops sardea (Hemiptera: Notonectidae) against larvae of Culex vishnui, vector of Japanease encephalitis, 6(30): 1-6 (doi: 10.5376/jmr.2016.06.0030)
To prevent emergence of insecticide resistance in vector population & biological magnification of toxic insecticides through food chain, more attention is now put on application of biological control system to reduce the population of vectors. Present study was carried out to assess the effect of habitat modifications on the predatory ability of Anisops sardea (Hemiptera: Notonectidae) against immature of Culex Vishnui (Diptera: Culicidae) group of mosquitoes in laboratory and semi-field bioassay.To study the effect of habitat modifications, laboratory based predatory experiments were carried with A. sardea as predator and Cx.vishnui group of mosquitoes as prey species. To determine prey preference of predator species, predatory experiment was done in presence of mosquitoes belongs to different genera. For a long-term study on predation rate, for 15 consecutive days, 10 adult individuals of A. sardea were kept in a plastic bucket of 20 L capacity in open natural condition and number of mosquito larvae consumed/per day was calculated.Result of single factor ANOVA analysis revealed that habitat modifications have significant effect on the predatory rate of A sardea as calculated F value (34.10) is significantly higher than table value (Fcrit=2.156). Pair wise ‘t test’ revealed significant variations in the predation rate when different habitat combinations were analyzed. Results of selectivity index, niche breadth and food breadth were also evaluated. Long term bioassay in semi-field condition revealed that predatory rates were almost similar throughout the study period.Considering the habitat specificity, prey preference and the rate of predation in semi field condition, it can be concluded that A. sardea can be used as potential biological control agent against Culex Vishnui group of mosquitoes.
Introduction
Mosquito is a holometabolous insect. Its life cycle includes: eggs hatched in water, an aquatic larval phase comprising of four instars, aquatic pupal phase and adults that moves from aquatic medium after its emergence from pupa. This type of holometabolous life cycle prevents aquatic larvae from competing with terrestrial/aerial adults because they inhabit different ecological niches. Due to the restricted habitat of larvae in aquatic medium and low rate of dispersal; most of the population regulation or biological control processes are recommended during this phase.
Prey-Predator interaction is one of the most important biological interactions in aquatic environments, which maintain the structure of the aquatic community. Many aquatic insect predators, like odonate larvae Bradynopyga geminate(Venkatesh et al., 2015 ; Varshini and Kanagappan,2014), Diplonychus rusticus and Anisops bouvieri (Brahma et al.,2014) , dytiscid beetles(Chandra et al.,2008),odonate naiads(Mandal et al.,2008),and the hemipteran bugs(Ghosh and Chandra,2011) are already documented by different researchers as good mosquito control agents in laboratory and field bioassay. Odonates are effective predators of mosquito larvae in aquatic habitat, particularly in temporary water bodies (Saha et al.,2012; Mogi,2007). They shows a high searching efficiency and good attack rate (Martínez and Castro, 2007), and mosquito larvae constitute the major part of their diet9. Similarly Notonectids, B. fuscipennis have also been reported for their mosquito larvivorous activity in structurally simple habitats where chironomids are abundant as alternative prey (Fisher et al.,2013). Mosquito species also avoid predator-inhabited oviposition sites by only detecting predator-released kairomones (Warburg et al.,2011;Himeidan et al., 2013). In a laboratory base experiment it was established that water bug and larvae of odonate species can effectively reduce mosquito density in the presence of multiple alternative prey (Saha et al.,2014).
Anisops sardea Herrich-Schaeffer (Hemiptera: Notonectidae) is a smaller-bodied aquatic backswimmer. It is very common in temporary pools and permanent water bodies in tropical countries including India. Its density in aquatic habitat reaches its maximum during rainy season (Konan et al.,2015). It has been proved by predatory experiment in a laboratory that, this species exhibits high predation rate against larval Culex mosquitoes(Tawfik et al.,1986). Effects of this particular predator on oviposition habitat selection of mosquitoes and other dipterans and on community structure due to their co- existence in similar aquatic habitat was investigated (Eitam et al.,2002). The functional responses of this species against Cx. quinquefasciatus., the most common filarial vector in South East Asia was also established (Mondal et al.,2014).
The objective of the present investigation is to study the effects of varied habitats on the predatory efficacies of this species in simulated natural condition established in the laboratory condition against Cx. vishnui group of mosquitoes. Present study also includes the study of selectivity index, niche breadth and food breadth of this predator against larval mosquitoes. The study also extended to include the status of predatory potential of A. sardea in long term bioassay experiment in semi-field condition.
1 Materials and Methods
Adults of A. sardea, were collected from the different temporary and permanent water bodies of Bankura town (23° 14' N, 87° 07' E) during May-June 2015. The surface of selected aquatic habitats were sieved by an insect net having 200-µm mesh size. Collected adults were temporarily stored in plastic containers in field and then transferred in glass aquarium in laboratory. Some aquatic weeds (Pistia sp, Hydrilla sp etc.) and gravels were placed inside the aquarium to produce natural conditions. From the collected samples, few specimens were identified upto species level in Zoological Survey of India. The average body lengths of the A. sardea was measured by an ocular micrometer with a scale. Predator species used in the experiments were 6 - 7mm in length. Mosquito larvae were collected from temporary aquatic habitat in rice fields of the same area at regular intervals during the experiments as required. After each collection, from the mixed population of mosquito larvae, the third instars larvae of Culex vishnui group were separated based on length and maturity and kept within enamel trays in the laboratory for 10 days before the laboratory bioassay for acclimatization with an adequate amount of artificial food (Tetra Bits Complete, German). The specific instars were identified by appropriate literatures (Chandra, 2000; Christophers, 1933). Third instars larvae were used during the experiments as they are easily visible and there is no chance to form a pupa unlike fourth instars. The predatory species were also acclimatized for 10 days with artificial food and Cx. vishnui group larvae.
To study the effect of varied habitat conditions on the predation rate of A. sardea, the predatory experiment was carried out in 1L of water with 100, 3rd instars larvae of Cx. vishnui group in each set. Different environmental determinants in laboratory experiments included presence of riverine water (collected from river Gandeswari), sludge water, pond water, tap water, vegetation (inclusion of 2 Hydrilla and 2 Pistia plants in glass beaker), 24h light, 24h dark and 12h dark: 12h light within a BOD incubator (Eastern Instrument, 180 cft) as experimental set up. In laboratory experiments, to show the effect of vegetation and light (24h light, 24h dark and 12h dark: 12h light) on predatory behavior of A.sardea, distilled water were used to avoid any interaction between habitat conditions. Nine replicates in each of the environmental conditions were carried out with separate adult morphs with similar size for determination of the rates of predation.
A total of 40 preys belongs to four different genera (10 Anopheles subpictus; 10 Culex vishnui group; 10 Armegeres subalbatus and 10 Stegomyia aegypti larvae) were placed in a 250 ml glass beaker. An adult individual of A. sardea was introduced into the beaker as predator. The predation rate of A. sardea was noticed for 24h to know the prey preference of the predator species. The result was analyzed according to Manly’s prey selectivity analysis(Manly et al.,1972; Krebs,1999; Rehage et al.,2005) The formulae used were:
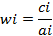
where, wi = preference to particular prey ci = proportion of the ith prey consumed and ai = proportion of the availability of ith prey
and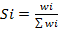
Wher, Si = selectivity index to particular prey species
The niche breadth (N) and food breadth (B) against prey items were also calculated. The formula used was,
N = 1/ Σ (ci2/ ai) and
B = (N-amin)/ (1 – amin) respectively.
where, amin is the lowest proportion of prey type available.
In the present study the niche breadth and Food breath was used to compare the difference in the predation rate of predator species against different mosquito genera as well to determine the utilization of food items when both prey and predators are available in same aquatic habitat.
For a long-term study on predation rate, for 15 consecutive days, 10 adult individuals of A. sardea were kept in a plastic bucket of 20 L capacity and placed in open natural condition. Some aquatic weeds such as Pistia sp and Hydrilla sp were introduced in the form of aquatic vegetation. 100 third instar larvae of Cx. vishnui group were given as food every day. After counting the consumed larva and pupal form each day, same number of larvae was introduced in the plastic bucket to maintain the same prey density every day. Three replicates were carried out during the study. The rate of predation was noted and the data obtained from the experiments was used to calculate the clearance rate (CR) using equation of the Gilbert and Burns(Gilbert and Burns,1999).
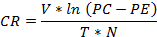
Where, V is the volume of water (in litres), T = time in hour, N = number of predators, PE = prey left after T time in the experiment and PC the prey at the start of the experiment.
2 Result
The results of changes in predation rates with habitat modifications have been presented in Fig.1. The result showed that predation rate was significantly changed in the presence of variable habitats. Highest rate of predation was noticed in 24h dark and the lowest rate was noticed in the presence of sludge water. Result of single factor ANOVA analysis and pair wise ‘t test’ between different habitat conditions on the predation rate of A.sardea has been presented in Table 1. It was observed that changes in the habitations have significant effect on the rate of predation of A. sardea as calculated F value (34.10) is significantly higher than table value (Fcrit=2.156). Results of pair wise ‘t test’ revealed there were some significant effects (p˂0.05) on rate of predation when different combinations are analyzed pair wise. For example, presence of sludge water and tap water showed a significant decrease in predation rate in all habitat combinations where as the combined effects of some parameters, viz. vegetation× 24hlight; vegetation× 24h dark; vegetation× (12h dark: 12h light); 24h light× 24h dark and 24h dark × (12h dark: 12h ligh) have no significant changes in the predation rate of A. sardea on Cx.vishnui Group.
|
Figure 1 Changes in the predation/consumption rates of A. sardea with habitat modifications on Cx. vishnui group |
|
Table 1 Results of Single factor ANOVA analysis and pair wise ‘ t test’ analysis between different habitat conditions pair wise to establish the effect of habitat modifications on the predation rate of A. sardea |
Niche breadth, selectivity index and food breadth are used to establish generalist predatory pattern of animals (Tyagi et al.,2015). Niche breadth is used to quantify niches in order to investigate potential competition between related species that shares a common habitat. Niche breadth has also been called niche width or niche size by ecologists. In the laboratory bioassay Selectivity index was calculated as 0.247 for St. aegypti, 0.267 for An. Subpictus, 0.288 for Cx. vishnui and 0.196 for Ar. Subalbatus. Results of ‘paired t test’ revealed that selectivity index was highest for Cx. vishnui group and it is significantly different (p<0.05) from Ar. subalbatus (t=6.396) but not different (p>0.05) from An. subpictus (t=1.532) and St. aegypti (t=2.431) against the table value of 2.687 at 4 degrees of freedom. Niche breath and Food breadth were calculated as 0.98 and 0.97 respectively.
The results of long term bioassay with Cx. vishnui group as prey and A.sardea as predator are presented in Table 2. The rates of predation of A. sardea during the course of long-term experiments remained almost similar during the entire set of experiments as expressed in terms of clearance rates, which reflected the ability of the predators to regulate the mosquito larvae in real time situations.
|
Table 2 Changes in predation rate and clearance rate (during interaction between A.sardea and Cx. vishnui group) during long term bioassay in semi-field condition for fifteen consecutive days (n=3 observations) |
3 Discussions
The results of the present study show that A. sardea is a good predator of mosquito larvae in laboratory condition. The selectivity index was high against all the three major vectors i.e. Cx. vishnui group, An. stephensi and Ae. aegypti. In the present study, it has been found that the both the values of Niche breadth and Food breadth are very close to 1 indicating the generalist predatory pattern of A.sardea in the presence of larvae of multiple mosquito species. Quantitative estimation of predation rates with changes in habitat revealed that the feeding rate although highest in dark condition but is almost similar in light and dark periods which definitely prove that these backswimmers use both vision and mechano receptors for capturing prey. The lower rate of predation in the presence of sludge water indicated that the polluted water caused decrease in vision and mechano-reception necessary for prey capture. A slight reduction of prey predation in presence of vegetation occurred probably due to the decreased space available for prey-predator interaction. Reduction of prey consumption also occurred in the presence of tap water due to the experimental set up in an unnatural habitat.
Clearance rate is an index of predation, which reflects the amount of time and space utilized by a predator in capturing, killing and consumption of a prey. The long term bioassay also indicates their predatory response against mosquito larvae in the semi field condition and it remained similar throughout the study period. From the observations on the prey-predator interaction between A.sardea and Cx. vishnui group of mosquitoes in semi-field condition, it can be predicted that effective regulation of mosquito immature can be achieved by application of adult morphs of A. sardea in field condition also.
Considering the habitat specificity, prey preference, the rates of predation in semi field condition during the present study, it can be concluded that of A. sardea can be used as potential biological control agent against any type of mosquito species (particularly Anopheles, Stegomyia and Cx. vishnui group) in clean temporary or permanent water logged aquatic habitats.
Authors’ contribution
RPM performed the Larvicidal bioassay experiment and systematized the data. AG performed the drafting of the manuscript.SB performed the mathematical interpretation of the data and GC supervised the whole work and approved the final manuscript.
Acknowledgements
We want to express our acknowledgement to The Director, Zoological Survey of India, Kolkata, for identification of the predator species. Rajendra Prasad Mondal want to acknowledge the financial support received from University Grants Commission (UGC), New [MRP Grant No. F. PSW-004/13-14 (ERO) ID No. WB1-009; S. No. 219563].
Brahma S., Aditya G., Sharma D., Saha N., Kundu M., and Saha G.K., 2014, Influence of density on intra guild predation of aquatic Hemiptera (Heteroptera): implications in biological control of mosquito, Journal of Entomological and Acarological Research, 46(1977):6-12
http://dx.doi.org/10.4081/jear.2014.1977
Chandra G., Mandal S.K., Ghosh A., Das D., Banerjee S.S., and Chakraborty S., 2008, Biocontrol of larval mosquitoes by Acilius sulcatus(Coleoptera: Dytiscidae)., BMC Infectious Diseases ,8:138 doi:10.1186/1471-2334-8-138
https://doi.org/10.1186/1471-2334-8-138
Chandra G., 2000, Mosquito, Sribhumi Publication Co., India and Kolkata pp.1-102
PMid:10946237
Christopher S.R.,1933, The Fauna of British India, including Ceylon and Burma: Diptera,Taylor and Francis, London
Eitam A., Blaustein L., Mangel M.,2002, Effects of Anisops sardea(Hemiptera: Notonectidae) on oviposition habitat selection by mosquitoes and other dipterans and on community structure in artificial pools, Hydrobiologia., 485:183–189
https://doi.org/10.1023/A:1021315309758 :
Fischer S., Zanotti G., Castro A., and Quirogal Vargas D.V., 2013,Effect of habitat complexity on the predation of Buenoa fuscipennis (Heteroptera: Notonectidae) on mosquito immature stages and alternative prey, Journal of Vector Ecology., 38(2):215-223:
https://doi.org/10.1111/j.1948-7134.2013.12033.x
Ghosh A., Chandra G., 2011,Functional responses of Laccotrephes griseus (Hemiptera: Nepidae) against Culex quinquefasciatus (Diptera: Culicidae) in laboratory bioassay, J Vector Borne Dis., 48:72– 77
Gilbert J.J., and Burns C.W., 1999, Some observations on the diet of the back swimmer Anisops Wakefieldi (Hemiptera:Notonectidae), Hydrobiologia., 412:111–118
https://doi.org/10.1023/A:1003812718853
Himeidan Y.E., Temu E.A. Rayah E.A.E1, Munga S., and Kweka E.J.,2013, Chemical Cues for Malaria Vectors oviposition: Site Selection:Challenges and Opportunities, Journal of Insects,1-9
http://dx.doi.org/10.1155/2013/685182
Konan K.l., Fofana D., Koné A.B., Konan Y.L., Assé H Kouassi D., Dosso M., Marsollier L., Aubry J., N'goran: K.E., and Doannio J.M.C.,2015, Inventory of aquatic heteroptera in ponds near the villages of six health districts, endemic to buruli ulcer in côte d'ivoire (west africa),The Experiment., 31(1):2012-2021
Krebs C.J.,1999, Ecological Methodology, Benjamin Cummings,Carlifornia: Mandal S.K., Ghosh A., Bhattacharjee I., and Chandra G., 2008, Biocontrol efficiency of Odonate nymphs against larvae of the mosquito, Culex quinquefasciatus Say 1823, ActaTropica.,106:109–114:
https://doi.org/10.1016/j.actatropica.2008.02.002
Manly B.F.J., Millar P., Cook M., 1972, Analysis of Selective Predation Experiment, The American naturalist.,106 (952):719-736
Martínez H.Q., and Castro A.R.,2007, Aquatic insects as predators of mosquito larvae, In: T.G. Floore (ed.) J. Am. Mosq. Contr. Assoc., 23 (Suppl. 2):110-117
Mogi M.,2007, Insects and other invertebrate predators, J. Am. Mosq. Contr. Assoc.,23(suppl 3): 93-109
https://doi.org/10.2987/8756-971X(2007)23[93:IAOIP]2.0.CO;2 :
Mondal R.M., Ghosh A., Chandra G.,2014,Functional response analysis of Anisops sardea (Hemiptera, Notonectidae) against Culex quinquefasciatus in laboratory condition, Indian Journal of Medical Research.,140 (4):551-555:
Rehage J.S., Barnetti B.K., and Sih A., 2005, Foraging behaviour and invasiveness: Do invasive Gambusia exhibit higher feeding rates and broader diets than their non invasive relatives?,Ecol. Freshwater Fishes.,14:352-360:
https://doi.org/10.1111/j.1600-0633.2005.00109.x
S Brahma S., Aditya G., Sharma D., Saha N., Kundu M., and Saha G.K., 2014, Influence of density on intra guild predation of aquatic Hemiptera (Heteroptera): implications in biological control of mosquito,Journal of Entomological and Acarological Research., 46(1977):6-12
Saha N., Aditya G., Banerjee S., and Saha G.K.,2012, Predation potential of odonates on mosquito: Implication for biological control, Biological Control ., 63:1-8
https://doi.org/10.1016/j.biocontrol.2012.05.004 :
Saha N., Aditya G., Saha G.K.,2014, Prey preferences of aquatic insects: potential implications for the regulation of wetland mosquitoes, Medical and Veterinary Entomology, 28:1-9:
https://doi.org/10.1111/mve.12003
Tawfik M.F.S., Husseini M.M.E.l.,and Bakr H.A.,1986,The biology of the notonectidae Anisops sardea H.S., an active mosquito predator in Egypt, Bull. Soc. Entomol.Egypte, 66: 117–126
Tyagi B.K., Munirathinam A., and Venkatesh A., 2015, A catalogue of Indian mosquitoes, International Journal of Mosquito Research.2 (2): 50-97
Varshini R.A., and Kanagappan M., 2014, Effect of quantity of water on the feeding efficiency of dragonfly Nymph –Bradynopyga geminata (Rambur), Journal of Entomology and Zoology Studies, 2 (6): 249-252
Venkatesh A., and Tyagi B.K.,2015, Bradinopyga geminata (Anisoptera: Libellulidae) as a predator of Aedes aegypti immature (Diptera: Culicidae), International Journal of Mosquito Research, 2 (2):98-105
Warburg A., Faiman R., Shtern A., Silberbush A., Markman S., Cohen J.E.,and Blaustein L.,2011, Oviposition habitat selection by Anopheles gambiae in response to chemical cues by Notonecta maculate, Journal of Vector Ecology,36(2):421-425
. PDF(0KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Anupam Ghosh
. Goutam Chandra
. Subhasis Bandyopadhyay
. Rajendra Prasad Mondal
Related articles
. Predator
. Culex vishnui group
. Bioassay
. Habitat modification
. Selectivity index
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)