Incidence of Malaria in Type 2 Diabetic patients and the effect on the liver: a case study of Bayelsa state 
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2016, Vol. 6, No. 15 doi: 10.5376/jmr.2016.06.0015
Received: 23 Mar., 2016 Accepted: 12 May, 2016 Published: 10 Jun., 2016
Ndiok E.O., Ohimain E.I., and Izah S.C., 2016, Incidence of malaria in type 2 diabetic patients and the effect on the liver: a case study of Bayelsa State, Journal of Mosquito Research, 6(15): 1-8 (doi: 10.5376/jmr.2016.06.0015)
Malaria and diabetics are common diseases especially in tropical countries. The study was carried out to assess the effect on the liver of Type 2 diabetics with malaria using Type 2 diabetics without malaria and non-diabetics with/without malaria as control subjects. Two hundred persons participated in this study, comprising of 100 diabetics (50 type 2 diabetics with malaria and 50 type two diabetics without malaria) and 100 non diabetics (50 non diabetics with malaria and 50 non diabetics without malaria). Samples was collected and analyzed for blood sugar, malaria parasite and liver function tests using standard procedures. Mean result for diabetics with malaria, diabetes without malaria, non-diabetes with malaria and non-diabetes without malaria were 4.47, 4.73, 5.16 and 4.46 mmol/L respectively (conjugated bilirubin), 19.04, 15.85 24.02 and 19.76 respectively iu/L (alkaline phosphatase), 39.78, 40.70, 40.05 and 38.10 g/L respectively (albumin), 68.90, 69.68, 69.18 and 71.06 g/L respectively (total protein), 5.96, 7.96, 7.32 and 6.24 mmol/L respectively (total bilirubin), 7.44, 6.08, 6.60 and 6.76 IU/L respectively (Alanine transaminase), 7.29, 6.69, 10.43 and 7.41 IU/L, respectively (Aspartate transaminase). The mean malaria parasite was 2376.83 and 2943.23 parasite numbers/μL for Type 2 diabetes and Non-diabetics patients, respectively. The Fasting blood glucose from the Type 2 diabetes and Non-diabetics patients was 6.92 and 3.89 mmol/L respectively. Analysis of variance showed that there were no significant differences (P>0.05) among the various treatment apart from alkaline phosphatase for liver function test. Also significant variation (P<0.05) exist for malaria parasite count and fasting blood sugar. All liver function parameters apart from alkaline phosphatase were within the normal range for both diabetics with and without malaria and non-diabetics with and without malaria. The increased alkaline phosphatase in both Type 2 diabetics and non-diabetics with malaria indicates that individuals with severe malaria parasitaemia and other complications such as diabetics may be at risk of liver impaired.
1 Introduction
Mosquito is iniquitous dipteran fly that are responsible for the transmission of many deadly diseases including malaria, filariasis, dengue, encephalitis, chikungunya, rift valley fever, yellow fever and other diseases which cause numerous deaths annually (Bhattacharya et al., 2014a; Bhattacharya et al., 2014b; Mukherjee et al., 2015). At as today, over 3,500 species of mosquitoes are found worldwide (Mallick et al., 2014; Dash et al., 2014), belonging to Diptera (Insecta) order and Culicidae family with three subfamilies including Anophelinae, Culicinae and Toxorhynchitinae (Dash et al., 2014). Generally, some species of mosquito could cause some viral diseases. For instance, in tropical and subtropical region especially in Asia, Aedes mosquitoes Aedes (Stegomyia) aegypti which are invasive in nature are typical vector of Dengue and Dengue Hemorrhagic Fever viruses (Saleem et al., 2014). Also, Culex quinquefasciatus, a culicine mosquito is a major vector of lymphatic filariasis which could also contribute to the transmission of avian malaria (Bhattacharya et al., 2014b).
Malaria is widely caused by mosquitoes of the Anopheles gambiae complex, including Anopheles arabiensis and Anopheles gambiae in Africa region (Hamza et al., 2014). Malaria is a major health issue. According to Akinneye and Afolabi (2014), malaria is the most prevalent of mosquito borne disease and is endemic in about 109 countries globally, where 190~330 million individuals are affected causing the death of about 1 million persons annually. Malaria is caused mainly by one-cell parasite of the genus Plasmodium and is transmitted from person to person mainly from the bite of a female Anopheles mosquito, mostly from 5 pm to 7 am, with maximum intensity between 10 pm and 4 am under Nigerian environmental condition (Ogundeyi et al., 2015). Typically, four species of Plasmodium causes malaria in humans including Plasmodium falciparum, P. ovale, P. malariae and P. vivax and in rare cases caused by Plasmodium knowlesi (Onyesom et al., 2012). Of these, Ogundeyi et al. (2015) reported that P. falciparum is the most virulent, common and severe in Africa particularly sub-Saharan region.
Malaria is endemic in Nigeria contributing to morbidity and mortality. The malaria incidence in Nigeria showed seasonal variation among the several of geopolitical coverage (Ogundeyi et al., 2015). For instance, FMOH (2001) cited in Ogundeyi et al. (2015) reported the incidence of malaria in the six geographical regions as 32.7% (South-South), 36.6% (South-West), 30.7% (South-East), 58.8% (North central), 55.3% (North East) and 33.6% (North West). Malaria typically affects all age groups of about 50% of the population on annual basis (Ogundeyi et al., 2015). Malarial infection disturbs metabolic and cellular behavior probably due to hepatic damage by perhaps the exoerythrocytic form of P. falciparum which inhabits the liver (Onyesom et al., 2012).
On the order hand, diabetes is a chronic metabolic disorder characterized by increased blood glucose levels due to inadequate endogenous insulin production by the pancreatic beta cells for type 1 diabetes (an autoimmune disease characterized by T-cell mediated destruction of the pancreatic beta cells) or by functioning of insulin secretion for type-2 diabetes (production of insulin resistance and sub normal functioning of the beta cell) (Ezuruike and Prieto, 2014; Al-Jameil et al., 2014). However, type 2 diabetes is also known as Non-Insulin-Dependent Diabetes Mellitus (Ikekpeazu et al., 2010). In recent time, the incidence of the risk factors and the prevalence of diabetics have been on the increasing trend globally but more in developing nations (Ezuruike and Prieto, 2014; Al-Jameil et al., 2014) especially in sub-Saharan African countries (Acquah et al., 2014), thereby contributing to morbidity and mortality in most nations across the globe (Arredondo, 2014). In 2010, over 12 million people in sub-Saharan Africa were diabetic leading to about 330,000 death from diabetes-related conditions and complications and it has been projected that incidence rate could reach 23.9 million by 2030 (Diabetes Leadership forum, 2010).
Both disease conditions (malaria and diabetes mellitus) are common in developing nations and are major killer diseases (Ikekpeazu et al., 2010). However, Acquah et al. (2014) noted that malaria and type 2 diabetes mellitus has continue to affect millions of people worldwide especially in developing countries and as such type 2 diabetes mellitus and malaria can be considered as a global phenomenon suggesting that developing nations separately or synergistically battle for type 2 diabetes mellitus and malaria. Due to this trend, there is the need to study the interaction between type 2 diabetes mellitus and malaria due to possible compounding health effects accrued to increased incidence of type 2 diabetes mellitus and the endemic nature of malaria in sub-Saharan African (Acquah et al., 2014).
Both malaria and type 2 diabetes mellitus diseases are frequent in developing nations such as Nigeria, there is need to assess the biochemical profile of type 2 diabetic patients with malaria parasitemia to know the possible contribution of malaria infection of the hepatic organ to the pathophysiology of diabetes mellitus (Ikekpeazu et al., 2010). According to Danquah et al. (2010), increased diabetes mellitus prevalence could put more persons at risk for malaria infection. Though information about this have been carried out by Ikekpeazu et al. (2010) in type 2 diabetic patients with malaria parasitaemia and without malaria parasitaemia attending the diabetic clinic of the University of Nigeria Teaching Hospital, Enugu State, Nigeria. Hence the present study focused on the patients attending diabetic clinic in Federal Medical Centre Yenagoa, Bayelsa state, Nigeria.
2 Material and Methods
2.1 Study area
Yenagoa is the capital of Bayelsa state, Nigeria. Bayelsa state has vast wetland and sedimentary basin characterized by depression. The state has several water bodies including river, creeks, creek lets etc, which empties into the Atlantic Ocean via River Nun (A major river). Typically, the surface water bodies of the state include fresh, brackish and marine. Fishing is a major occupation of the inhabitants of the state especially in the rural areas. The region often suffer from urban flooding hence the incidence of mosquito bites is prevalent. At as 2006 census, the population is about 1.7 million people. But have increased significantly within the last 10 years due to urbanization, industrialization and migration.
2.2 Study population
The study population was made up of two hundred (200) males and females. Their ages varied from 18~40 years and compromising of fifty (50) diabetic males and females with Malaria parasitaemia, Fifty (50) diabetic males and females without Malaria parasitaemia, Fifty (50) non-diabetic males and females with Malaria parasitaemia, Fifty (50) non-diabetic males and females without Malaria parasitaemia. The study was carried out in Federal Medical Centre (FMC), Yenagoa, Bayelsa state between May and July 2015. The experimental protocol was approved by the Ethics Committee of the Federal Medical Centre, Yenagoa, Bayelsa state. Also the patients were notified.
2.3 Exclusion criteria
Children and pregnant females were not recruited. Also, adults with known hepatic disease conditions (hepatitis etc) were not recruited.
2.4 Sample collection
Sample collection was carried out at the Medical laboratory services reception by a phlebotomist. The upper arm was wrapped with elastic band (tourniquet) and the vein was cleaned with alcohol prior to blood collection. Using 5 mL disposable syringes was pierced into the vein. Thereafter the band was removed from subject’s arm when enough blood was collected. Then a cotton ball was put over the needle site as the needle was removed. Pressure was put on the site and then a bandage put on. The blood was dispensed into plain containers for the liver function tests, fluoride-oxalate containers for fasting blood sugar and into EDTA containers for malaria parasite tests.
2.5 Laboratory methods and procedures
For Aspartate Aminotransferase, alkaline phosphatase, Alanine Aminotransferase, total protein and albumin were determined using spectrophotometer (RT-9200 semi-auto chemistry analyzer). The serum protein was estimated quantitatively using Biuret Method as modified by randox Laboratories (United Kingdom) for protein analysis in body fluid and the one recommended by the international Federation of Clinical Chemistry expert panel for the determination of total protein (Tietz, 1995) at a wavelength of 546 nm. The Serum Aspartate Aminotransferase and Alanine Aminotransferase were estimated quantitatively using the concentration of oxaloacetate hydrazone formed as modified by randox Laboratories (United Kingdom) (Schmidt and Schmidt, 1963) at a wavelength 546 nm. Serum alkaline phosphatase was estimated quantitatively using colorimetric endpoint method by Teco Diagnostics Laboratories (USA) (Roy, 1970) at 630 nm wavelength. Serum albumin was estimated quantitatively using Bromocresol Green as modified by randox Laboratories (United Kingdom) (Tietz, 1987) at 630 nm wavelength. Total and conjugated bilirubin was estimated quantitatively using colorimetric method as modified by Randox Laboratories (United Kingdom) at a wavelength of 600 nm using digital automated analyzer (Selectra ProS).
The blood glucose was estimated using glucose oxidase method of Trinder and the malaria parasite test was carried out using the method described by Cheesbrough (2005). A thick and thin blood films were made and stained using Giemsa stain for 30 minutes and the films were allowed to dry. The slides were viewed under the microscope using the 100× objective, which were examined at 100 high power microscope fields. Malaria density was estimated by counting parasites against white blood cells. The number of parasites per microliter (μL) of blood was calculated as follows:
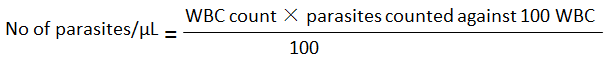
WBC = white blood count
2.6 Statistical analysis
SPSS software was used to carry out the statistical analysis. The data were expressed as Mean±standard error. Statistical analyses were carried out using the Student’s t-test (P<0.05), and followed by one-way analysis of variance carried out at P=0.05 and Duncan Multiple Range Test was used for multiple comparisons between more than two treatment/groups.
3 Result and Discussion
Two hundred persons participated in this study, comprising of 100 diabetics (50 type 2 diabetics with malaria and 50 type two diabetics without malaria) and 100 non diabetics (50 non diabetics with malaria and 50 non diabetics without malaria).
The fasting blood glucose level and malaria parasite number in blood of type 2 diabetes and Non-diabetics patients attending diabetic clinic in Federal Medical Center, Yenagoa Bayelsa state are presented in Table 1. The mean malaria parasite was 2,376.83 and 2,943.23 parasite numbers/μL for type 2 diabetes and Non-diabetics patients respectively. There was significance difference (P<0.05) among the two class of patients. Lower malaria parasite counts in diabetics patients could be due to the fact that human insulin suppresses the mosquito immune system; hence mosquito immunological resistance to malaria reduces its spread among humans (American Society of Microbiology, 2012, www.sciencedaily.com/releases/2012/06/120626061206.htm). The level of parasite reported in this study is lower than the value of 103.9 number/mL and 164.4 count/mL in diabetics with malaria and non-diabetes with malaria respectively from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu as reported by Ikekpeazu et al. (2010). Also, the findings of this study is far lower to the report of Mohapatra (2001), who reported 2,058.2 and 4,560 parasite count/mL for severe falciparum malaria from diabetic with malaria and non-diabetic with malaria patient. The lower value obtained from this study as compared to previous study could be due to the level of parasites in the blood and the medication that have been taken prior to the various sampling. The lower relative malaria parasite counts in diabetic patients could be due to slow multiplication. A study in Ghana, Danquah et al. (2010) provides evidence out of 1,466 urban patients with type 2 diabetes mellitus 46% have increased risk for infection with Plasmodium falciparum. However, the real reasons for the increase of P. falciparum infection are uncertain (Danquah et al., 2010). Hence, the increased risk factor with regard to glucose level is an indication of biologic plausibility and such risk could result from dysfunctional defense against liver and/or blood-stage parasites and from prolonged persistence (Danquah et al., 2010).
.png) Table 1 Fasting blood glucose and Malaria parasite numbers/μl of blood samples in Type 2 Diabetic and Non- Diabetics. Data is expressed as Mean ± Standard Error; Data in parenthesis are range; Different letter as superscript across the row indicate significant difference (P<0.05). |
The Fasting blood glucose from the Type 2 diabetes and Non-diabetics patients was 6.92 and 3.89 mmol/L respectively. There was significance difference (P<0.05) among the two class of patients. Again the variation in both classes of patients could be due to insulin resistance in type 2 diabetes (Ezuruike and Prieto, 2014). The blood glucose level observed in this study is slightly higher than the values of 8.94 and 4.93mmol/L for diabetics with malaria and non-diabetes with malaria patients respectively attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu as reported by Ikekpeazu et al. (2010). Also, the findings of this study is close to the report of Mohapatra (2001), who reported blood glucose from diabetic with malaria and non-diabetic with malaria as 110.4 mg/dL (6.11 mmol/L) and 76.2 mg/dL (4.23mmol/L) respectively. Also Al-Jameil et al. (2014) reported similar trend of result with fasting blood glucose of 13.6 mmol/L and 3.7mmol/L for diabetic and non-diabetic patients. Also, Kayode et al. (2011) reported a value of 83.771 mg/dL) and 115.395 mg/dL (which translate to 4.65 mmol/L and 6.41mmol/L) for malaria and non-malaria patients from febrile patients. Hence the variation could be due to the intensity of the febrile disease. The blood glucose for non- diabetic patient was within the standard reference range of 2.5~5.6 mmol/L used in Federal Medical Center Yenagoa, Bayelsa state. This is a clear indication that they are really diabetic. Though, some of the type 2 diabetic patient’s glucose level was low as indicated in the range (1.40~17.10 mmol/L). This could be attributed to the fact that the patients are on insulin therapy, which aid to regulate their blood glucose level.
Table 2 presents parameters for liver health in diabetics with and without malaria and non-diabetics with and without malaria in patients attending diabetic clinic in Federal Medical Center, Yenagoa Bayelsa state, Nigeria. The mean of alkaline phosphatase values are 24.02 (non-diabetics with malaria), 19.76 (non-diabetes without malaria), 19.04 (diabetes with malaria) and 15.85 (diabetes without malaria). Basically there were significant differences (P<0.05) among the various treatment. The findings of the study are lesser than previous report. Ikekpeazu et al. (2010) reported alkaline phosphatase value of 59.10 IU/L (non-diabetics with malaria), 55.5 IU/L (non-diabetes without malaria), 59.30 IU/L (diabetes with malaria) and 55.7 IU/L (diabetes without malaria) from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu, Nigeria. In this study, apart from non-diabetes with malaria patients, the values obtained were lesser than the reference range of 20~140 IU/L used in Federal Medical Center Yenagoa. Typically, alkaline phosphatase showed an elevation that was significant. These were observed for both the type 2 diabetics with malaria and non-diabetics with malaria. Higher alkaline phosphatase in diabetics with malaria could be due to enhancement in the hepatic stage of the parasite’s life cycle in its host, which is frequently accompanied by significant perturbation of the hepatocyte membrane, thereby leading to enzyme escape from the liver cells (Adekunle et al., 2007). Therefore, alkaline phosphate activity is a potential indicator for hepatic drainage system in acute P. falciparum malarial infection (Onyesom et al., 2013). Increased alkaline phosphatases have been reported in humans (Onyesom and Onyemakonor, 2011) and animal model (Adekunle et al., 2007). The increase in alkaline phosphate is because of the membrane bound enzyme that could trigger the reaction of several phosphate esters especially in the liver which is one of the major site (Adekunle et al., 2007). Onyesom et al. (2013) listed sources of alkaline phosphatase to include liver, bone, kidney etc. But the cause of low alkaline phosphate in this study is unknown.
.png) Table 2 Comparisons of liver health parameters in diabetics with and without malaria and non-diabetics with and without malaria. Each value is expressed as mean±standard error (n=50). The same letters in each row indicate no significant differences at P> 0.05 according to the Duncan Multiple Range Test (DMRT) Statistics. |
The mean albumin values of 40.05 g/L (non-diabetics with malaria), 38.10 g/L (non-diabetes without malaria), 39.78 g/L (diabetes with malaria) and 40.70 g/L (diabetes without malaria). There were no significant difference (P>0.05) among the various treatment. The parameters for liver health of the test subjects (treatment) are within normal range for albumin in humans. The range of values obtained was within the reference range of 35~50 g/L used in Federal Medical Center Yenagoa. Higher albumin was observed for patient with malaria. This could increase catabolism of albumin leading to nitrogen loss (Adekunle et al., 2007). Diabetic with malaria have lower albumin compared to diabetics without malaria though not significant. Typically malaria infections with attendant pathology reduce albumin in the serum, which is synthesized in the liver (Kayode et al., 2011).
The mean total protein values for diabetics with malaria, diabetes without malaria, non-diabetes with malaria and non-diabetes without malaria were 68.90 and 69.68, 69.18 and 71.06 g/L respectively. There was no significant variation (P>0.05) among the various treatment. The parameters for liver health of the test subjects (treatment) are within normal range for total protein in humans. The range of values obtained was within the reference range of 58~80 g/L used in Federal Medical Center Yenagoa. However, protein is lower in subject with malaria which is in consonance with the findings of Adekunle et al. (2007) of Plasmodium yeolli in model animal. The lower concentration of protein in patients with malaria could be due to the fact that serum protein is synthesized in the liver and malaria attack the liver (Adekunle et al., 2007).
The mean total bilirubin values of 7.32 mmol/L (non-diabetics with malaria), 6.24 mmol/L (non-diabetes without malaria), 5.96 mmol/L (diabetes with malaria) and 5.96 mmol/L (diabetes without malaria). There were no significant difference (P>0.05) among the various treatment. The parameters for liver health of the test subjects (treatment) are within normal range for total bilirubin in humans, thus 1.71~17.1 mmol/L stipulated for normal human in reference range from Federal Medical Center, Yenagoa. The finding of this study is slightly lower than the observation of Ikekpeazu et al. (2010), who reported total bilirubin as 9.55 mmol/L (non-diabetics with malaria), 8.97 mmol/L (non-diabetes without malaria), 11.10 mmol/L (diabetes with malaria) and 9.99 mmol/L (diabetes without malaria) from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu, Nigeria.
The mean conjugated bilirubin values for diabetics with malaria, diabetes without malaria, non-diabetes with malaria and non-diabetes without malaria were 4.47, 4.73, 5.16 and 4.46 mmol/L, respectively. There was no significant variation (P>0.05) among the various treatments. The liver health parameters of the test subjects (treatment) are within normal range for conjugated bilirubin in humans. Thus, the range in the various treatment groups is within the reference range of 1.7~8.5 mmol/L used in Federal Medical Center Yenagoa. The findings of this study are close to report from previous study. Ikekpeazu et al. (2010) reported conjugated bilirubin of 4.74 mmol/L (non-diabetics with malaria), 4.21 mmol/L (non-diabetes without malaria), 5.33mmol/L (diabetes with malaria) and 4.78 mmol/L (diabetes without malaria) from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu, Nigeria.
The mean alanine transaminase values for diabetics with malaria, diabetes without malaria, non-diabetes with malaria and non-diabetes without malaria were 7.44, 6.08, 6.60 and 6.76 IU/L, respectively. There was no significant difference (P>0.05) among the various treatments. The parameters for liver health of the test subjects (treatment) are within normal range for Alanine transaminase in humans which is up to 12 IU/L based on the reference range used in Federal Medical Center Yenagoa. The trend of result of this study is slightly lower than the findings of Ikekpeazu et al. (2010), who reported Alanine transaminase value of 8.40 IU/L (non-diabetics with malaria), 7.95 IU/L (non-diabetes without malaria), 8.00 IU/L (diabetes with malaria) and 7.40 IU/L (diabetes without malaria) from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu, Nigeria. Al-Jameil et al. (2014) reported similar trend for diabetic and non-diabetic patients with Alanine transaminase value of 57.1 u/L and 20.3 u/L for diabetic and non-diabetic patients, respectively.
The mean aspartate transaminase values for diabetics with malaria, diabetes without malaria, non-diabetes with malaria and non-diabetes without malaria were 7.29, 6.69, 10.43 and 7.41, respectively. There was no significant difference (P>0.05) among the various treatments. The parameters for liver health of the test subjects (treatment) are within normal range for aspartate transaminase in humans. The liver health function of the test subjects are within normal range of up to 12 IU/L for aspartate transaminase in humans based on the reference range used in Federal Medical Center Yenagoa. The result of this study is similar to that of Ikekpeazu et al. (2010), who reported aspartate transaminase value of 9.8 IU/L (non-diabetics with malaria), 10.6 IU/L (non-diabetes without malaria), 10.8 IU/L (diabetes with malaria) and 10.3 IU/L (diabetes without malaria) from patient’s attending diabetic clinic at University of Nigeria Teaching Hospital, Enugu, Nigeria. Al-Jameil et al. (2014) also reported similar trend for diabetic and non-diabetic patients with aspartate transaminase value of 51.5 u/L and 16.9 u/L for diabetic and non-diabetic patients, respectively.
The apparent variation in aspartate transaminase, alanine transaminase, conjugated bilirubin and total bilirubin could be due to compounding factors, such as drugs being taken by patients, immune system strength etc. Generally, the trend of this result was comparable to the findings of Ikekpeazu et al. (2010), who reported that there was significant difference in alkaline phosphate among the treatments but no variation in other liver function tests including total bilirubin, conjugated bilirubin, alanine transaminase, aspartate transaminase.
4 Conclusion
Type 2 Diabetes Mellitus and Malaria have continually been shown to be the major health challenges common in major developing countries. The present study evaluates the possible contribution of malaria infection of the liver to the pathophysiology of type 2 diabetes mellitus. The study found that diabetics with malaria and those without malaria showed normal liver function although alkaline phosphatase was slightly raised and significant in both type 2 diabetics and non-diabetics with malaria. Both type 2 diabetics and non-diabetics with malaria showed slight increase in alkaline phosphatase, signifying that patients with severe malaria parasitaemia and other complications such as diabetics may be at higher risk of liver in discharge of its functions.
Acknowledgements
This publication is based on the Master of Science project work of the lead author (Eno Okon Ndiok) and supervised by the second author (Dr Elijah Ige Ohimain) at the Niger Delta University, Nigeria. The author also wish to thank the two anonymous reviewers of the manuscript for their valuable suggestion and comments.
Acquah S., Boampong J.N., Eghan Jnr B.A., and Eriksson M., 2014, Evidence of Insulin Resistance in Adult Uncomplicated Malaria: Result of a Two-Year Prospective Study, Malaria Research and Treatment, 2014: 136148-136148
http://dx.doi.org/10.1155/2014/136148
Adekunle A.S., Adekunle O.C., and Egbewa B.E., 2007, Serum status of selected biochemical parameters in malaria: an animal model, Biomedical Research, 18 (2): 109-113
Akinneye J.O., and Afolabi O.J., 2014, Toxicity and fumigant effect of powder and oil extracts of cleisthopholis patens (benth) againstlarvae and adults anopheles mosquito, Journal of Mosquito Research, 4(11): 1-6
Al-Jameil N., Khan F.A., Arjumand S., Khan M.F., and Tabassum H., 2014, Associated liver enzymes with hyperlipidemic profile in type 2 diabeties patients, International Journal of Clinical and Experimental Pathology, 7(7): 4345-4349
Arredondo A., 2014, Type 2 diabeties and health care costs in Latin America: exploring the need for greater preventive medicine, Bmc Medicine, 12(1): 1-6
http://dx.doi.org/10.1186/s12916-014-0136-z
Bhattacharya K., Burman S., Nandi S., Roy P., Chatterjee D., and Chandra G., 2014, Phytochemical extractions from the leaves of Ravenala adagasariensis from Sundarban area and its effect on southern house mosquito (Culex quinquefasciatus Say 1823) larvae, Journal of Mosquito Research, 4(12): 1-6
Bhattacharya K., Chandra I., Kundu P., Ray S., Halder D., and Chandra G., 2014, Larval control of Culex vishnui group through bio-active fraction of traveller’s tree, Ravenala madagascariensis Sonn. (Strelitziaceae), Journal of Mosquito Research, 4(15): 1-6
Cheesbrough M., 2005, District laboratory practice in tropical countries, 2nd Edition Cambridge University press, pp. 239-258
http://dx.doi.org/10.1017/CBO9780511581304
Danquah I., Bedu-Addo G., and Mockenhaupt F.P., 2010, Type 2 Diabetes mellitus and increased risk for malaria infection, Emerging Infectious Diseases, 16(10):1601-1604
http://dx.doi.org/10.3201/eid1610.100399
Dash S., Hazra R.K., and Bisht S.S., 2014, Investigation on the mosquito fauna of shoreline habitats of orissa coast, India, Journal of Mosquito Research, 4(20): 1-5
Ezuruike U.F., and Prieto J.M., 2014, The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations, Journal of Ethnopharmacology, 155(2): 857-924
http://dx.doi.org/10.1016/j.jep.2014.05.055
FMOH, 2001, National strategic plan for Roll Back Malaria in Nigeria 2001 -Abuja: Federal Ministry of Health, Nigeria
Hamza A.M., EI-Rayah E.A., and Abukashawa S.M.A., 2014, Molecular characterization of mosquitoes of anopheles gambiae species complex (Diptera: Culicidae) from Sudan and Republic of Southern Sudan, Journal of Mosquito Research, 4(13): 1-10
Ikekpeazu E.J., Neboh E.E., Maduka I.C., Nwagbara I.J., and Nwobodo M.W., 2010, Type-2 diabetes mellitus and malaria parasitaemia: effect on liver function tests, Asian Journal of Medical Sciences, 2(5): 214-217
Kayode O.T., Kayode A.A.A., and Awonuga O.O., 2011, Status of selected hematological and biochemical parameters in malaria and malaria-typhoid co-infection, Journal of Biological Science, 11(5): 367-373
http://dx.doi.org/10.3923/jbs.2011.367.373
Mallick S., Bhattacharya K., and Chandra G., 2014, Mosquito larvicidal potentiality of wild turmeric, Curcuma aromatic rhizome, extracts against Japanese Encephalitis vector Culex vishnui group, Journal of Mosquito Research, 4(19): 1-6
Mohapatra M.K., 2001, Profile of severe falciparum malaria in diabetics, International Journal of Diabetics in Developing Countries, 21: 156-161
Mukherjee A., Chatterjee D., Patra S., Mandal B., and Ghosh A., 2015, Differences in community perceptions on mosquito borne diseases between rural and urban localitities of Bankura District, West Bengal, India, Journal of Mosquito Research, 5(1): 1-5
Ogundeyi S.B., Idowu O.A., Fadairo J.K., and Daniels A.O., 2015, Prevalence of malaria amongst children 0-4 years in Olugbo, Odeda local government, Ogun State, Nigeria, Journal of Mosquito Research, 5(16): 1-4
Onyesom I., and Onyemakonor N., 2011, Levels of parasitaemia and changes in some liver enzymes among malarial infected patients in Edo-Delta region of Nigeria, Current Research Journal of Biological Sciences, 3(2): 78-81
Onyesom I., Osioma E., and Aguele M.O., 2013, Placental alkaline phosphatase activity in serum of some Nigerian pregnant women infected with malaria, Frontiers in Science, 2(6): 200-202
http://dx.doi.org/10.5923/j.fs.20120206.11
Roy A.V., 1970, Rapid method for determining alkaline phosphatase activity inserum with thymolphthalein monophosphate, Clinical Chemistry, 16(5): 431-436
Saleem M., Ghouse G., Hussain D., Saleem H.M., and Abbas M., 2014, Distribution of dengue vectors during pre-and post-monsoon seasons in three districts of punjab, Pakistan, Journal of Mosquito Research, 4(16): 1-5
Schmidt E., and Schmidt F.W., 1963, Determination of serum GDT and GPT activities. Biologica et Clinica, 3: 1-53
Tietz N.W., 1987, Fundamentals of clinical chemistry,Saunders, p328-329
Tietz N.W., 1995, Estimation of serum alanine amino transferase. In clinical guide to laboratory tests, 3rd edition. W.B. Saunders Philadelphia, p76
. PDF(269KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Ndiok O.
. Ohimain I.
. Izah C.
Related articles
. Diabetics
. Human Health
. Liver
. Malaria
. Mosquitoes
Tools
. Email to a friend
. Post a comment