Research Report
Bioactivity of Acyranthes aspera (Amaranthaceae) Foliage against the Japanese Encephalitis Vector Culex vishnui Group 
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2013, Vol. 3, No. 13 doi: 10.5376/jmr.2013.03.0013
Received: 24 Sep., 2013 Accepted: 10 Oct., 2013 Published: 05 Nov., 2013
Kuntal Bhattacharya and Goutam Chandra, 2013, Bioactivity of Acyranthes aspera (Amaranthaceae) Foliage against the Japanese Encephalitis Vector Culex vishnui Group, Journal of Mosquito Research, Vol.3, No.13 89-96 (doi: 10.5376/jmr.2013.03.0013)
Background and objective: Failure to develop proper vaccines against mosquito borne diseases, a global health problem, imposes sole reliance on the vector managerial steps for reducing the disease incidences. Easy abundance, cost effectiveness, target specificity as well as bio-degradability of botanicals draw the most attention as vector control agents than their synthetic counterparts which facilitate vector resistance and intoxicate natural resources. The present study estimated larvicidal activities of the crude and solvent extracts of Acyranthes aspera against the vector of Japanese encephalitis Culex vishnui group under laboratory conditions.
Methods: Crude extracts of A. aspera foliage ranging from 0.1% to 0.5% concentrations were examined for larvicidal activity against 1st to 4th instars larvae of Cx. vishnui group. Extractions of the active fractions were carried out by means of six different solvents in a non-polar to polar approach viz. petroleum ether, n-hexane, ethyl acetate, chloroform: methanol (1:1 v/v), acetone, and absolute alcohol. Dose dependent mortality was established through graded concentrations ranging from 20 ppm to 100 ppm using the bioactive fractions. Further, determinations of LC50 and LC90 values of crude and bioactive fractions were accomplished through log-probit analyses. Statistical justifications of the larvicidal property were established through ANOVA analyses regarding instars, time and concentrations as three completely randomized independent variables. Costing impacts on the non-target water fauna of the bio-active portion were assessed under laboratory conditions.
Result: In a 72 hour bioassay experiment with crude extract, the highest mortality was recorded in 0.5% concentration. Acetone extractive was found to exert efficient larvicidal activity amongst all the solvent extractives. Cent per cent mortalities were exhibited by 1st and 2nd instars larvae at 48 hours of exposure while 3rd instars larvae showed 97.32% mortality at 72 hours of exposure with LC50 value of 32.15 ppm. An obvious dose-dependent mortality was established through regression analyses, as the rate of mortality (Y) was positively correlated with the concentration (X). The non-target populations were primarily non-responsive to plant extracts under study.
Conclusion: Extract of A. aspera foliage is of great consequence having appreciable larvicidal activity against Cx. vishnui group. The compound is environment friendly and largely non-toxic to non-target organisms.
Introduction
Mosquitoes, hematophagous dipteran flies, toil as the chief vector for the transmission of malaria, filariasis, dengue fever, yellow fever, schistosomiasis, Japanese encephalitis and many more noxious diseases pertaining to numerous deaths annually. Mosquito bite induces both immediate (types I and III) and delayed (type IV) hypersensitivity responses (Clements, 1992) leading to the development of angioedema, intense itching, redness and swellings in humans. In tropical country like India, over 40 million people get infected each year (Ghosh et al., 2012) with mosquito borne diseases. Amongst these, Japanese encephalitis, prevalent in Southeast Asia and the Far East, is caused by Japanese encephalitis (JE) virus belonging to flaviviridae family. Around 3 billion natives of the world population subsist in JE-endemic regions while 15,000 deaths out of more than 50,000 cases per annum in Indian sub-continent alone (Kabilan et al., 2004) are recorded. Domestic pigs and wild birds act as natural reservoir hosts for this deadly virus. Isolation of JE virus from diverse mosquito species from tropics has made it possible to scientifically conclude that culicine mosquitoes mainly Culex vishnui group (Culex vishnui, Cx. pseudovishnui and Cx. tritaeniorhynchus) is the principal vector of JE virus (Colless, 1957). The proverbial Cx. vishnui group is pervasive and tenderly breeds in paddy fields (Banerjee and Chandra, 2004) and other water bodies with luxuriant vegetation. Their profusion is directly related to the increase in rice cultivation, shallow ditches and pools and that’s why India, a major rice cultivating nation, is highly endemic for JE. The situation is more likely to be shoddier as pig rearing is growing exponentially along with augmentation in both cropping sector and cropping intensity (Keiser et al., 2005). Thus control of Cx. vishnui group is need of the hour to minimize the socio-economic crises.
Several synthetic insecticides have been developed and employed in the field with considerable success to control the vector but, they are proved to be bio-magnifying toxic hazards due to their non-selectivity, residual exposure, and non-biodegradability (Wattal et al., 1981). Along with these, resistance to pesticides (Tabashnik, 1994) has drawn attention to find out novel insecticides and study of naturally occurring insecticides especially from plant origin (Rawani et al., 2009; Chowdhury et al., 2009) that are eco-friendly and target specific than their synthetic equivalents.
Achyranthes aspera, a common weed plant of Amaranthaceae family, is distributed throughout the tropical world. It is perennial around 25~118 cm tall having quadrangular, juvenile with slightly puffed up nodes and contradictory branching pattern (Srivastav et al., 2011). The hairy surface of leaf bears elliptic-oblong blade like structure and papery in texture. The plant is known to exhibit significant abortifacient activity in rabbits and mice and is found to acquire contraceptive competence in rats pertaining to its potent estrogenicity. Concerning our literature review, no information is presented on its larvicidal effect on Cx. vishnui group so far.
The purpose of present study was to evaluate the larvicidal activity of the crude and solvent extracts of A. aspera leaves against Cx. vishnui group as target species.
1 Results
The differential yields of all the solvent extracts through Soxhlet extraction from 200 g of leaves were as follows: petroleum ether extract, 6.73 g; n-hexane extract, 2.78 g; ethyl acetate extract, 3.40 g; chloroform: methanol (1:1, v/v) extract, 5.38 g; acetone extract, 2.70 g; and absolute alcohol extract, 2.66 g. The consequences of the study pointed out that the mortality rate of the larvae was highest at 0.5% concentration of the crude extract when tested against all the larval instars. The mortality percentages of the all the instars with crude extract treatment were presented in Table 1. Log probit analyses clearly indicated augmentations in LC50 (Figure 1) and LC90 (Figure 2) values (at 95% confidence level) concerning larvicidal effects of later stages larvae which were subsequently subordinated with increase in exposure period (from 24 hours to 72 hours).
.png) Table 1 Dose response larvicidal assay using crude extract of A. aspera foliage against Cx. vishnui group |
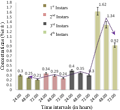 Figure 1 Graphical presentation of LC50 values of crude extract of A. aspera foliage against Cx. vishnui group |
 Figure 2 Graphical presentation of LC90 values of crude extract of A. aspera foliage against Cx. vishnui group |
The larval mortality of 3rd instars using all the different solvent was presented in Table 2. Acetone extract was found to be the best larvicide, through several trials, among all the solvent extracts of A. aspera leaves. The larval mortality of Cx. vishnui group using acetone extractives was depicted in Table 3 regarding all the instars, differential time exposure and concentration gradient as three random variables. Highest mortality was achieved at 100 ppm of concentration. The LC50 and LC90 values of acetone extract were determined through log-probit and regression analyses and presented in Table 4. The larvicidal effect of acetone extractives against Cx. vishnui group were found to be statistically significant when the mortality rate (Y) was correlated with the concentration of exposure (X) positively with regression coefficient (R2) close to 1 in each case (Table 4). However the non-target populations were nonresponsive i.e., no changes in the survival rate and swimming activity, when exposed to 47.30 ppm concentration of acetone extract i.e., LC50 value of 3rd instars for a period of 24 hours. The larvicidal activity of A. aspera leaves were found statistically significant (p < 0.05) through completely randomized ANOVA analyses (Table 5).
Table 2 Percent mortality of 3rd instars larvae of Cx. vishnui group using all the six solvents at 72 hours of post-exposure |
.png) Table 3 Dose response larvicidal assay using acetone extract of A. aspera foliage against Cx. vishnui group |
.png) Table 4 Assessment of LC50 and LC90 values of acetone extract of A. aspera through log-probit and regression analyses |
Table 5 Completely randomized three way ANNOVA analysis using concentration (C), hour (H) and instars (I) as three parameters |
2 Discussion
Employment of eco-friendly and biodegradable natural insecticides of plant origin has been recognized and given importance, recently, for mosquito borne disease control. Larval control is regarded as the best approach to diminish mosquito population at very early stage as imprisonment to water bodies and very stumpy rate of scattering make the mosquito larvae most susceptible. Hence, mosquito control is mostly aimed at wrigglers’ control and only against matures when necessary. Botanicals are proved to be efficient bio-pesticides not only as crude extract but as solvent extracts also. Diverse plant ingredients are verified to be larvicidal (Bhattacharya and Chandra, 2013, Kundu et al., 2013), pupicidal (Rawani et al., 2012), adulticidal, repellent and smoke toxic (Chowdhury et al., 2007) against different mosquito species. The current study well documented the bio-activity of crude extract of A. aspera foliage against Cx. vishnui group. Adhikari et al. (2012) established the larvicidal activity of crude extract of Swietenia mahagoni leaves against Cx. vishnui where cent percent mortality of 3rd instars was recorded at 0.4% concentration following an exposure period of 72 h with LC50 and LC90 values of 0.05% and 0.28% correspondingly. However, in the present study 78.68% mortality of 3rd instars was achieved followed by 72 hours of post exposure at 0.5% concentration with significant (p < 0.05) elevated values of LC50 (Figure 1) and LC90 (Figure 2). Among all the active fractions the acetone extract of A. aspera showed highest mortality at 100 ppm doses at 72 hour against Cx. vishnui group. Singha et al. (2012) also established the larvicidal effect of acetone extracts of Holoptelea integrifolia against Cx. vishnui group where cent percent mortality was achieved at 400 ppm concentrations. The larvicidal effect of the present study was significantly higher (p < 0.05) at relatively low concentration (100 ppm) of active fraction. The larvicidal property of essential oils of A. aspera against the early fourth instars were reported against Aedes aegypti by Khandagle et al (2011) with the LC50 and LC90 values of 761 ppm and 817 ppm respectively with an exposure period of 24 hours. The LC50 and LC90 value of the 4th instars larvae after 24 hours of post exposure were significantly (p < 0.05) lower in case of Cx. vishnui (Table 4).
In brief, the findings of the current investigation reveal that the leaf extract of A. aspera exhibits remarkable larvicidal activity against mosquitoes of Cx. vishnui group. Further laboratory investigations are required for illuminating the actual chemical compound responsible for larvicidal activity. This activity may again be tested against an extensive range of mosquito species in field conditions. Further investigations are also required to identify the chemical personality of the active ingredient and to successfully utilize, if possible, by formulating a commercial product.
3 Material and Methods
3.1 Compilation of plant materials
Fresh foliage of A. aspera were collected randomly during June 2012 from Golapbag Campus, The University of Burdwan (23°16´N, 87°54´E), West Bengal, India.
3.2 Rearing the larvae and colony set up
Assorted larvae Cx. vishnui group were collected from inundated rice fields of Agriculture Farm, The University of Burdwan (23°16´N, 87°54´E) through standard scooping and dipping method (Robert et al., 2002) to build up the colony. The larvae were housed in plastic trays filled with tap water and periodically fed with a mixture of Brewer yeast, dog biscuits and algae in 3:1:1 ratio (Kamaraj et al., 2011). Pupae were relocated from the trays to insectary (45×45×40 cm) where adults came out. In glass cages adults were nurtured and provided with 10% sucrose solution with multivitamin syrup in a container with a cotton wick. The mosquitoes were identified based on the key provided by Barraud (1934), Christophers (1933) and Chandra (2000). The adults of Cx. vishnui group were reared selectively in a different glass cage where, on the 5th day of rearing, adults (females) were given a blood meal from a non-motile shaved pigeon overnight. Petri dishes filled with 100 ml of tap water and wrinkled with filter paper were kept inside the cage for oviposition. The eggs were unruffled and allowed to hatch under laboratory conditions. The processes were repeated to set up a laboratory reared colony of Cx. vishnui group. The colony was maintained at (27±2)℃ temperature and 80%~85% relative humidity (RH) under a 13:11 light-and-dark cycles.
3.3 Crude extract procurements
Fresh and young green leaves of A. aspera were rinsed in tap water followed by distilled water and soaked on a paper towel. Then the unsoiled and unspotted leaves were pulverized by mechanical grinder and the liquid was filtered by Whatman’s no-1 filter paper. The filtrate was regarded as a stock solution (100% concentration) for the bioassay experiment.
3.4 Differential solvent extraction
For solvent extraction, fresh and clean leaves of A. aspera were dried for few days in shed. 200 g dried leaves of A. aspera were put into the column of the Soxhlet apparatus while 2 lit solvent was laden into the solvent chamber following 1:10 ratio. Six different solvents in a non-polar to polar fashion viz. petroleum ether, n-hexane, ethyl acetate, chloroform: methanol (1:1 v/v), acetone, and absolute alcohol were passed through the column one after another. The extraction period was fixed for 72 hours with 8 hours maximum a day for each solvent. Elutes were collected from the solvent chamber and concentrated through evaporation in a rotary evaporator. The extractives were preserved at 4℃ in a refrigerator.
3.5 Larvicidal bioassay
Trusting the WHO standard protocol (WHO/ VBC, 1981) the larvicidal bioassay was executed at the Mosquito and Microbiology Research Units, Parasitology Laboratory, The University of Burdwan. Twenty five larvae of precise stage from the laboratory reared larval colony were re-located in Petri-dishes of 9 cm diameter (150 mL capacity) and filled with 100 mL of tap water. Crude extractives were added in a graded concentrations ranging from 0.1% to 0.5% in different Petri dishes while 20 ppm to 100 ppm solvent extracts were taken in different Petri dishes for executing larvicidal bioassay. Each experiment was completed in triplicate (n=75) with a set of controls. Petri dishes were kept at room temperature (28 ± 2)℃, within (88±2)% relative humidity range for a total observation period of 72 hours. The larvae were assumed dead when they didn’t exhibit any movement by the pricking with a sharp needle in the siphon or cervical region or they were unable to achieve the water surface (Macedo et al., 1997). The no. of deceased wrigglers was calculated every 24 hours period up to 72 hours and percentage mortality was recorded from the average value of three replicates. The mortality data of 48 h and 72 h were expressed by additions of the mortalities of 24 h and 48 h, correspondingly. Firstly all the solvent extractives were screened against 3rd instars larvae and then the experiment was elaborated with the best active fraction against all the instars.
3.6 Costing the impacts on non-target populations
The little creatures sharing the same environment with mosquitoes are considered as the most fatal risk group. Vulnerability of these organisms to leaf extractives was figured out on Chironomus circumdatus larvae (insect). They were exposed to concentration level of LC50 value of 24 h of 3rd instars larvae to examine the mortality and other irregularities such as tardiness of swimming activity up to 72 h of exposure.
3.7 Statistical analysis
The percentage mortalities (%M) were precised by Abbott’s formula (Abott WS, 1925) during the observation of larvicidal potentiality of the plant extracts. Judicious determination of LC50 and LC90 values of crude and solvent extracts were carried out through Log-probit and regression analyses. Further statistical justifications were elaborated through ANOVA analyses using different instars, hours and different concentrations as three random variables to validate the significance between the above parameters and larval mortality.
Acknowledgements
The authors are indebted to Professor Dr. A. Mukhopadhyay, Botany Department, The University of Burdwan, for his kind help in plant species identifications. We are grateful to UGC DRS and DST-INSPIRE for providing financial assistance.
References
Abott W.S., 1925, A method of computing the effectiveness of an insecticide. J. Econ. Enotomol., 18(2): 265-267
Adhikari U., Singha S., and Chandra G., 2012, In vitro repellent and larvicidal efficacy of Swietenia mahagoni against the larval forms of Culex quinquefasciatus Say, Asian Pac. J. Trop. Biomed., s260-s264
Banerjee A., and Chandra G., 2004, Role of some factors on the breeding of JE vector Culex vishnui group, J. Commun. Dis., 36(4): 260-263
PMid:16506548
Barraud P.J., 1934, The Fauna of British India, including Ceylon and Burma, Diptera Vol –IV, Taylor and Francis London, pp.1-455
Christophers S.R., 1933, The Fauna of British India, including Ceylon and Burma, Diptera Vol -V. Taylor and Francis London, pp.360
Bhattacharya K., and Chandra G., 2014, Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrata L. (Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae), Asian. Pac. J. of Trop. Dis., 4(Suppl 1): S226-S232
Chandra G., 2000, Mosquito, Sribhumi Publication Co., pp.1-102
PMid:10946237
Chowdhury N., Bhattacharjee I., Laskar S., and Chandra G., 2007, Efficacy of Solanum villosum Mill. (Solanaceae: Solanales) as a biocontrol agent against fourth instar larvae of Culex quinquefasciatus Say, Turk. J. Zool., 31(4): 365-370
Chowdhury N., Chatterjee S.K., Laskar S., and Chandra G., 2009, Larvicidal activity of Solanum villosum Mill (Solanaceae: Solanales) leaves to Anopheles subpictus Grassi (Diptera: Culicidae) with effect on non-target Chironomus circumdatus Kieffer (Diptera: Chironomidae), J. Pest Sci., 82: 13-18
http://dx.doi.org/10.1007/s10340-008-0213-1
Clements Alan, 1992, The biology of mosquitoes – volume 1: Development, Nutrition and Reproduction, London, Chapman & Hall, ISBN 0-85199-374-5
Colless D.H., 1957, The Culex vishnui group (Diptera, Culicidae), with descriptions of two new species, Annals Trop. Med. Parasitol., 51(1): 87-101
PMid:13425320
Ghosh A., Chowdhury N., and Chandra G., 2012, Plant extracts as potential mosquito larvicides, Indian J. Med. Res., 135(5): 581-598 PMid:22771587
PMCid:PMC3401688
Kabilan L., Rajendran R., Arunachalam N., Ramesh S., Srinivasan S., and Samuel P.P., 2004, Japanese encephalitis in India: An overview, Indian J. Paediatr., 71: 609-615
http://dx.doi.org/10.1007/BF02730659
http://dx.doi.org/10.1007/BF02724120
PMid:15280610
Kamaraj C., Bagavan A., Elango G., Zahir A.A., Rajakumar G., Marimuthu S., Santhoshkumar T., and Rahuman A., 2011, Larvicidal activity of medicinal plant extracts against Anopheles subpictus &Culex tritaeniorhynchus, Indian J. Med .Res., 134(1): 101-106
PMid:21808141 PMCid:PMC3171902
Keiser J., Maltese M.F., Erlanger T.E., Bos R., and Tanner M., 2005, Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management, Acta Trop, 95: 40-57
http://dx.doi.org/10.1016/j.actatropica.2005.04.012
PMid:15878762
Khandagle A.J., Tare V.S., Raut K.D., and Morey R.A., 2011, Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes, Parasitol Res.,109: 339-343, DOI 10.1007/s00436-011-2261-3
http://dx.doi.org/10.1007/s00436-011-2261-3
Kundu M., Rawani A., and Chandra G., 2013, Evaluation of mosquito larvicidal activities of seed coat extract of Cassia sophera L., J. Mosq. Res., 3(11): 76-81
Macedo M., Consoli R.A.G.B., Grandi T.S.M., Des Anjos A.M.G., De Olivira A.B., Mendes M.M., Queiroz R.O., and Zani C.L., 1997, Screening of Asteraceae (Compositae) plant extract for larvicidal activity against Aedes fluviatilis (Diptera : Culicidae), Mem. Inst. Osw. Cruz., 92: 565-570
http://dx.doi.org/10.1590/S0074-02761997000400024
PMid:9361755
Rawani A., Mallick Haldar K., Ghosh A., and Chandra G., 2009, Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae), Parasitol. Res., 105: 1411-1417
http://dx.doi.org/10.1007/s00436-009-1573-z
PMid:19644705
Rawani A., Ghosh A., Laskar S., and Chandra G., 2012, Aliphatic amide from seeds of Carica papaya as mosquito larvicide, Pupicide, adulticide, repellent and smoke toxicant, J. Mosq. Res., 2(2)
http://dx.doi.org/10.5376/jmr.2012.02.0002
Robert V., Goff G.L., Ariey F., and Duchemin J.B., 2002, A possible alternative method for collecting mosquito larvae in rice fields, Malaria Journal, 1: 4
http://dx.doi.org/10.1186/1475-2875-1-4
Singha S., Adhikari U., Ghosh A., and Chandra G., 2012, Mosquito larvicidal potentiality of Holoptelea integrifolia leaf extract against Japanese encephalitis vector, Culex vishuni group, J. Mosq. Res., 2(4): 25-31
Srivastav S., Mishra G., Jha K.K., and Khosa R.L., 2011, Achyranthes aspera-An important medicinal plant: A review, J. Nat. Prod. Plant Resour., 1(1): 1-14
Tabashnik B.E., 1994, Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol., 39: 47-79
http://dx.doi.org/10.1146/annurev.en.39.010194.000403
Wattal B.L., Joshi G.C., and Das M., 1981, Role of agriculture insecticides in precipitating vector resistance, J. Commun. Dis., 13: 71-73
PMid:6119342
World Health Organization, 1981, Instructions for determining the susceptibility of resistance of mosquito larvae to insecticides, WHO/ VBC. 81.807: 1-6
. PDF(160KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Kuntal Bhattacharya
. Goutam Chandra
Related articles
. Acyranthes aspera
. Larvicide
. Culex vishnui group
. Non-target organism
Tools
. Email to a friend
. Post a comment